Antioxidant Activities of Phenolic Metabolites from Flemingia philippinensis Merr. et Rolfe and Their Application to DNA Damage Protection
- PMID: 29614846
- PMCID: PMC6017228
- DOI: 10.3390/molecules23040816
Antioxidant Activities of Phenolic Metabolites from Flemingia philippinensis Merr. et Rolfe and Their Application to DNA Damage Protection
Abstract
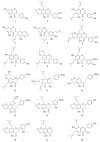
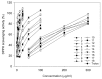
F. philippinensis Merr. et Rolfe has been cultivated on a large scale and is widely consumed by local inhabitants as an important nutraceutical, especially against rheumatism which has a deep connection with antioxidants. In this study, a total of 18 different phenolic metabolite compounds in F. philippinensis were isolated and identified, and evaluated for their antioxidant and DNA damage protection potential. The antioxidant activity of the 18 identified compounds was screened using DPPH, ORAC, hydroxyl and superoxide radical scavenging assays. The antioxidant potential of the compounds was found to differ by functionality and skeleton. However, most compounds showed a good antioxidant potential. In particular, seven of the identified compounds, namely, compounds 2, 3, 6, 10, 11, 15 and 16, showed significant protective effects on pBR322 plasmid DNA against the mutagenic and toxic effects of Fenton's reaction. The most active compound, compound 2, displayed a dose-dependent DNA damage protection potential in the range of 7.5~60.0 μM. The DNA damage protective effect of the identified compounds was significantly correlated with the hydroxyl radical scavenging activity. Compounds that exhibited effective (IC50 = 5.4~12.5 μg/mL) hydroxyl radical scavenging activity were found to be the ones with higher DNA damage protection potential.
Keywords: DNA damage protective effects; Flemingia philippinensis; electron spin resonance spectroscopy; phenolic metabolites; radical scavenging activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

