Anti-inflammatory effect of Mongolian drug Naru-3 on traumatic spinal cord injury and its mechanism of action
- PMID: 29614905
- PMCID: PMC6023071
- DOI: 10.1177/0300060518760157
Anti-inflammatory effect of Mongolian drug Naru-3 on traumatic spinal cord injury and its mechanism of action
Abstract
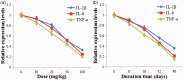
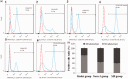
Objective This study was performed to confirm the anti-inflammatory effect of the Mongolian drug Naru-3 on traumatic spinal cord injury (TSCI) and its possible mechanism of action. Methods We prepared a TSCI model using Sprague-Dawley rats. The rats were divided into a Naru-3 group and a methylprednisolone group. Real-time polymerase chain reaction and western blotting were performed to measure the expression levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β. Enzyme-linked immunosorbent assay kits were employed to detect serum inflammatory cytokine levels. The localization and expression of monocyte chemotactic protein-1 (MCP-1) in spinal cord tissue was determined by immunohistochemical analysis. Flow cytometry was performed to analyze the ratio of M1- and M2-phenotype macrophages. The locomotor function recovery was evaluated by the Basso, Beattie, and Bresnahan score. Results Naru-3 significantly inhibited the inflammatory response and reduced the expression of TNF-α, IL-6, and IL-1β in both spinal cord and blood in a time- and concentration-dependent manner. Immunohistochemical analysis indicated that Naru-3 significantly reduced MCP-1 expression in spinal cord and promoted M2-phenotype macrophage differentiation. Conclusions Naru-3 is an effective treatment for impact-induced TSCI in rats. Naru-3 treatment affects inflammatory cytokine levels and macrophage differentiation, which play a role in TSCI remission.
Keywords: Naru-3; inflammatory cytokines; macrophages; methylprednisolone; monocyte chemotactic protein-1; traumatic spinal cord injury.
Figures




Similar articles
-
Minocycline-Loaded Poly(α-Lipoic Acid)-Methylprednisolone Prodrug Nanoparticles for the Combined Anti-Inflammatory Treatment of Spinal Cord Injury.Int J Nanomedicine. 2022 Jan 7;17:91-104. doi: 10.2147/IJN.S344491. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35027828 Free PMC article.
-
Isorhamnetin promotes functional recovery in rats with spinal cord injury by abating oxidative stress and modulating M2 macrophages/microglia polarization.Eur J Pharmacol. 2021 Mar 15;895:173878. doi: 10.1016/j.ejphar.2021.173878. Epub 2021 Jan 14. Eur J Pharmacol. 2021. PMID: 33453223
-
Geniposide exerts protective effects on spinal cord injury in rats by inhibiting the IKKs/NF-κB signaling pathway.Int Immunopharmacol. 2021 Nov;100:108158. doi: 10.1016/j.intimp.2021.108158. Epub 2021 Sep 20. Int Immunopharmacol. 2021. PMID: 34555642
-
[Effect of Herba Lycopodii Alcohol Extracted Granule Combined Methylprednisolone on Expression Levels of BDNF and NMDA and Behavior of Traumatic Spinal Cord Injury Rats].Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015 Aug;35(8):1004-10. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015. PMID: 26485919 Chinese.
-
Cytokine transport across the injured blood-spinal cord barrier.Curr Pharm Des. 2008;14(16):1620-4. doi: 10.2174/138161208784705450. Curr Pharm Des. 2008. PMID: 18673204 Free PMC article. Review.
Cited by
-
Interaction between piperine and genes associated with sciatica and its mechanism based on molecular docking technology and network pharmacology.Mol Divers. 2021 Feb;25(1):233-248. doi: 10.1007/s11030-020-10055-9. Epub 2020 Mar 4. Mol Divers. 2021. PMID: 32130644 Free PMC article.
-
Antipruritic effects of electroacupuncture on morphine-induced pruritus model mice through the TLR2/4-MyD88-NF-κB pathway.Neuroreport. 2019 Mar 20;30(5):331-337. doi: 10.1097/WNR.0000000000001203. Neuroreport. 2019. PMID: 30822282 Free PMC article.
References
-
- Wyndaele M, Wyndaele JJ. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord 2006; 44: 523–529. - PubMed
-
- Ning GZ, Yu TQ, Feng SQ, et al. Epidemiology of traumatic spinal cord injury in Tianjin, China. Spinal Cord 2011; 49: 386–390. - PubMed
-
- Lee BB, Cripps RA, Fitzharris M, et al. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord 2014; 52: 110–116. - PubMed
-
- Nashmi R, Fehlings MG. Changes in axonal physiology and morphology after chronic compressive injury of the rat thoracic spinal cord. Neuroscience 2001; 1041: 235–251. - PubMed
-
- Bethea JR, Dietrich WD. Targeting the host inflammatory response in traumatic spinal cord injury. Curr Opin Neurol 2002; 153: 355–360. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

