First molecular detection and characterization of Marek's disease virus in red-crowned cranes (Grus japonensis): a case report
- PMID: 29615025
- PMCID: PMC5883596
- DOI: 10.1186/s12917-018-1437-9
First molecular detection and characterization of Marek's disease virus in red-crowned cranes (Grus japonensis): a case report
Abstract
Background: Marek's disease virus (MDV) resides in the genus Mardivirus in the family Herpesviridae. MDV is a highly contagious virus that can cause neurological lesions, lymphocytic proliferation, immune suppression, and death in avian species, including Galliformes (chickens, quails, partridges, and pheasants), Strigiformes (owls), Anseriformes (ducks, geese, and swans), and Falconiformes (kestrels).
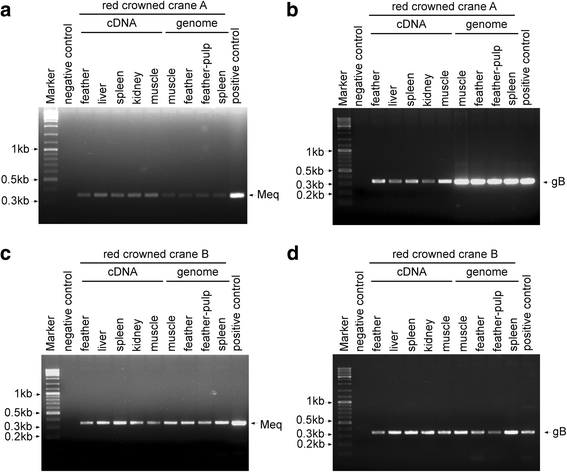
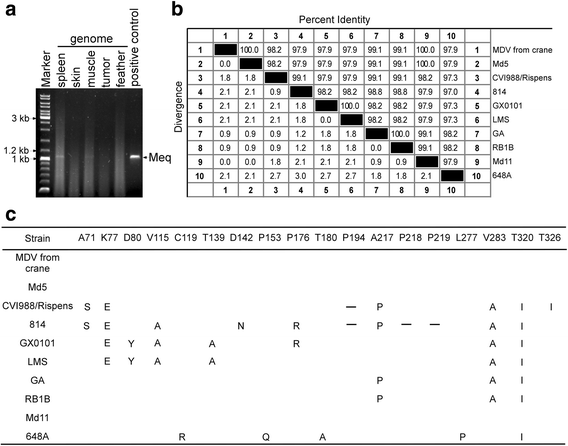
Case presentation: In 2015, two red-crowned cranes died in Nanjing (Jiangsu, China). It was determined that the birds were infected with Marek's disease virus by histopathological examination, polymerase chain reaction (PCR), gene sequencing and sequence analysis of tissue samples from two cranes. Gross lesions included diffuse nodules in the skin, muscle, liver, spleen, kidney, gizzard and heart, along with liver enlargement and gizzard mucosa hemorrhage. Histopathological assay showed that infiltrative lymphocytes and mitotic figures existed in liver and heart. The presence of MDV was confirmed by PCR. The sequence analysis of the Meq gene showed 100% identity with Md5, while the VP22 gene showed the highest homology with CVI988. Furthermore, the phylogenetic analysis of the VP22 and Meq genes suggested that the MDV (from cranes) belongs to MDV serotype 1.
Conclusion: We describe the first molecular detection of Marek's disease in red-crowned cranes based on the findings previously described. To our knowledge, this is also the first molecular identification of Marek's disease virus in the order Gruiformes and represents detection of a novel MDV strain.
Keywords: Clinical necropsy; Homology; Marek’s disease virus; PCR; Red-crowned crane.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the author’s institution (Nanjing Agricultural University ethics committee) and owner consent was obtained for the animals used for the post-mortem examinations.
Consent for publication
Consent was obtained from the owner of the animal for publication of this case report.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Grus japonensis:The IUCN Red List of Threatened Species 2016. http://www.iucnredlist.org/details/full/22692167/0.
-
- Collar NJ, Andreev A, Chan S, Crosby M, Subramanya S, Tobias J, Srinivasan C, Subramanya HS, Awatramani N, Subramanya HM. Threatened birds of Asia: the BirdLife international red data book. Cambridge (RU): Birdlife international; 2001.
-
- Kanai Y, Ueta M, Germogenov N, Nagendran M, Mita N, Higuchi H. Migration routes and important resting areas of Siberian cranes (Grus leucogeranus) between northeastern Siberia and China as revealed by satellite tracking. Biol Conserv. 2002;106(3):339–346. doi: 10.1016/S0006-3207(01)00259-2. - DOI
-
- Su L, Zou H. Status, threats and conservation needs for the continental population of the red-crowned crane. Chinese Birds. 2012;3(3):147–164. doi: 10.5122/cbirds.2012.0030. - DOI
-
- Wang Q. Threats for red-crowned crane. China Crane News. 2008;12(2):7–12.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

