An in vitro investigation of telocytes-educated macrophages: morphology, heterocellular junctions, apoptosis and invasion analysis
- PMID: 29615057
- PMCID: PMC5883889
- DOI: 10.1186/s12967-018-1457-z
An in vitro investigation of telocytes-educated macrophages: morphology, heterocellular junctions, apoptosis and invasion analysis
Abstract
Background: Telocytes (TCs), a recently discovered novel type of interstitial cells, were also found in a wide variety of human and mammalian reproductive organs/tissues, including uterus, oviduct and placenta. Previously, we demonstrated that TCs-conditioned media was capable of activating peritoneal macrophages (pMACs) through paracrine effects. This study investigates the hypothesis that direct interaction of TCs with pMACs will also play a significant role in immunoregulation of pMACs.
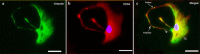
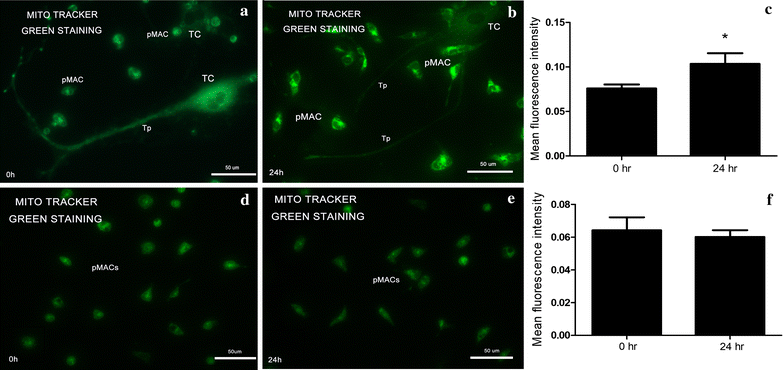
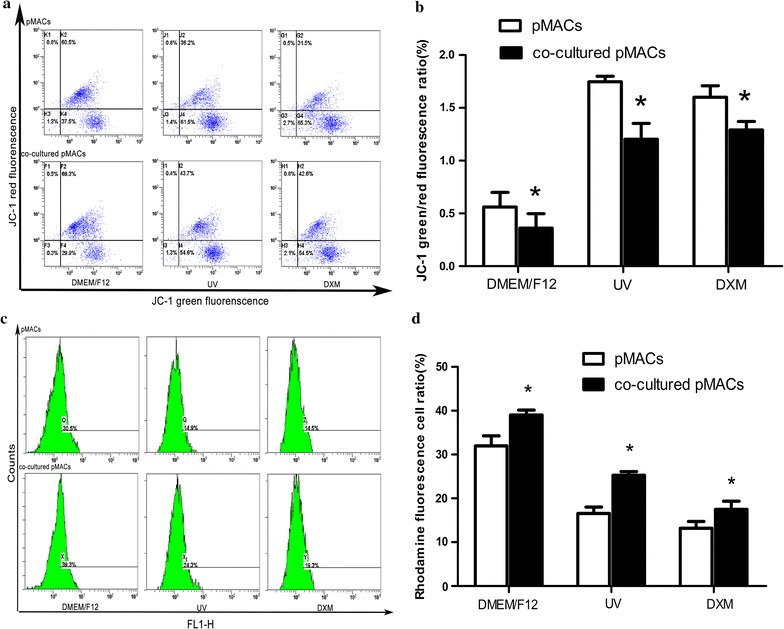
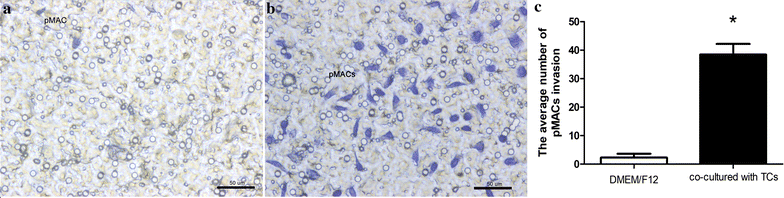
Methods: TCs and pMACs were derived from the uterus and intraperitoneal cavity of female BALB/c mice, respectively. TCs were identified by immunofluorescence and then co-cultured directly with pMACs for 24 h without added cytokines, to observe the in vitro biological behavior of pMACs. We used histochemical staining to study morphology and mitochondrial metabolism of pMACs, scanning electron microscopy to study heterocellular junctions, flow cytometry to investigate mitochondrial membrane potential (ΔΨm) and apoptosis, and transwell chambers to study invasion ability. Student-t test was used accordingly.
Results: Presently, TCs with typical structure and immunophenotype of double CD-34-positive/vimentin-positive were successfully isolated. pMACs co-cultured with TCs showed obviously morphological activation, with enhanced energy metabolism (P < 0.05). Meanwhile, direct physical cell-to-cell interaction promoted the development of heterocellular junctions between TCs and pMACs. Furthermore, TCs treatment markedly reduced the depletion of ΔΨm in co-cultured pMACs (all P < 0.05), and inhibited their apoptosis (P < 0.05). Functionally, pMACs co-cultured with TCs showed enhanced invasion ability (P < 0.05).
Conclusions: Direct physical cell-to-cell interaction promoted the development of heterocellular junctions between TCs and pMACs, presumably responsible for the observed novel efficient way of pMACs activation via mitochondrial signaling pathway. TCs-educated pMACs might be a promising way to restore the defective immunosurveillance in endometriosis (EMs), led to the enhanced treatment efficacy of EMs in a simple and clinically feasible fashion.
Keywords: Apoptosis; Endometriosis (EMs); Heterocellular junctions; Immunoregulation; Interstitial cells; Macrophage activation; Mitochondrial membrane potential (ΔΨm); Peritoneal macrophages (pMACs); Telocytes (TCs).
Figures



Similar articles
-
In vitro morphology, viability and cytokine secretion of uterine telocyte-activated mouse peritoneal macrophages.J Cell Mol Med. 2015 Dec;19(12):2741-50. doi: 10.1111/jcmm.12711. Epub 2015 Oct 16. J Cell Mol Med. 2015. PMID: 26471943 Free PMC article.
-
Telocytes Enhances M1 Differentiation and Phagocytosis While Inhibits Mitochondria-Mediated Apoptosis Via Activation of NF-κB in Macrophages.Cell Transplant. 2021 Jan-Dec;30:9636897211002762. doi: 10.1177/09636897211002762. Cell Transplant. 2021. PMID: 33787355 Free PMC article.
-
Ultrastructure damage of oviduct telocytes in rat model of acute salpingitis.J Cell Mol Med. 2015 Jul;19(7):1720-8. doi: 10.1111/jcmm.12548. Epub 2015 Mar 6. J Cell Mol Med. 2015. PMID: 25753567 Free PMC article.
-
Telocytes in Inflammatory Gynaecologic Diseases and Infertility.Adv Exp Med Biol. 2016;913:263-285. doi: 10.1007/978-981-10-1061-3_18. Adv Exp Med Biol. 2016. PMID: 27796894 Review.
-
Telocytes in the reproductive organs: Current understanding and future challenges.Semin Cell Dev Biol. 2016 Jul;55:40-9. doi: 10.1016/j.semcdb.2016.03.018. Epub 2016 Mar 25. Semin Cell Dev Biol. 2016. PMID: 27021165 Review.
Cited by
-
Morphological changes of telocytes in camel efferent ductules in response to seasonal variations during the reproductive cycle.Sci Rep. 2019 Mar 14;9(1):4507. doi: 10.1038/s41598-019-41143-y. Sci Rep. 2019. PMID: 30872789 Free PMC article.
-
Telocytes constitute a widespread interstitial meshwork in the lamina propria and underlying striated muscle of human tongue.Sci Rep. 2019 Apr 10;9(1):5858. doi: 10.1038/s41598-019-42415-3. Sci Rep. 2019. PMID: 30971762 Free PMC article.
-
Telocytes enhanced in vitro decidualization and mesenchymal-epithelial transition in endometrial stromal cells via Wnt/β-catenin signaling pathway.Am J Transl Res. 2020 Aug 15;12(8):4384-4396. eCollection 2020. Am J Transl Res. 2020. PMID: 32913513 Free PMC article.
-
Telocytes in the Normal and Pathological Peripheral Nervous System.Int J Mol Sci. 2020 Jun 17;21(12):4320. doi: 10.3390/ijms21124320. Int J Mol Sci. 2020. PMID: 32560571 Free PMC article. Review.
-
A Two-Step Immunomagnetic Microbead-Based Method for the Isolation of Human Primary Skin Telocytes/CD34+ Stromal Cells.Int J Mol Sci. 2020 Aug 16;21(16):5877. doi: 10.3390/ijms21165877. Int J Mol Sci. 2020. PMID: 32824287 Free PMC article.
References
-
- Roatesi I, Radu BM, Cretoiu D, Cretoiu SM. Uterine telocytes: a review of current knowledge. Biol Reprod. 2015;93(10):11–13. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

