Efficacy, safety and recurrence of new progestins and selective progesterone receptor modulator for the treatment of endometriosis: a comparison study in mice
- PMID: 29615065
- PMCID: PMC5883298
- DOI: 10.1186/s12958-018-0347-9
Efficacy, safety and recurrence of new progestins and selective progesterone receptor modulator for the treatment of endometriosis: a comparison study in mice
Abstract
Background: Current medical treatments for endometriosis are very limited. Progestin and selective progesterone receptor modulators (SPRM) are developed but their efficacy, safety, mechanism and recurrence in endometriosis are not fully studied.
Methods: In order to compare therapeutic, side effects and therapeutic actions of Esmya, Duphaston and Dienogest in endometriosis. Experimental endometriosis was induced by either intraperitoneal or subcutaneous mouse endometrium transplantation. Lesion size, weight and histology at the end of intervention were compared. Expression of related markers in the endometriotic lesions were examined. Body, uterus and ovary weights, endometrial glands and thickness (ETI), and follicle count were measured. For recurrent study, lesion growth before and after intervention was monitored.
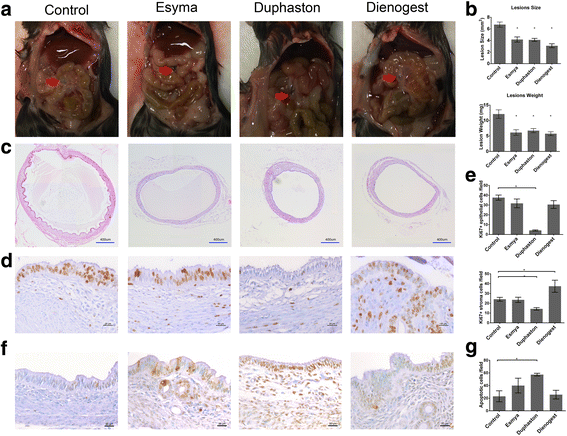
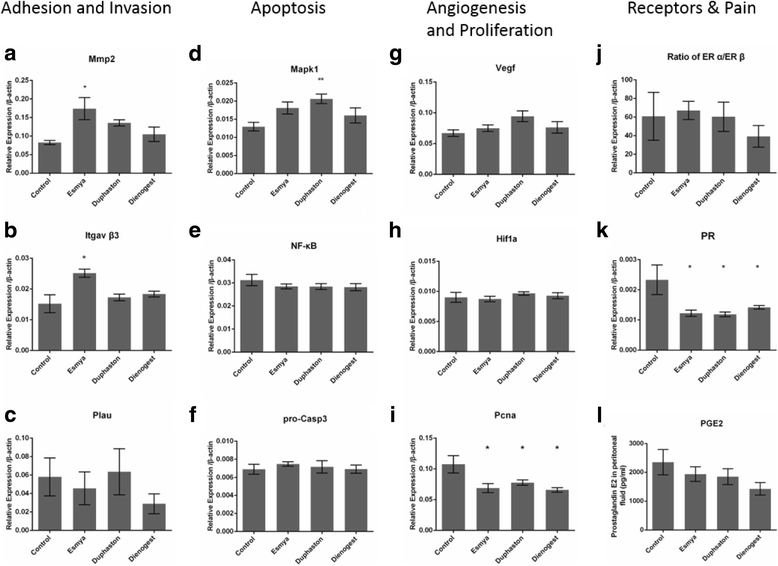
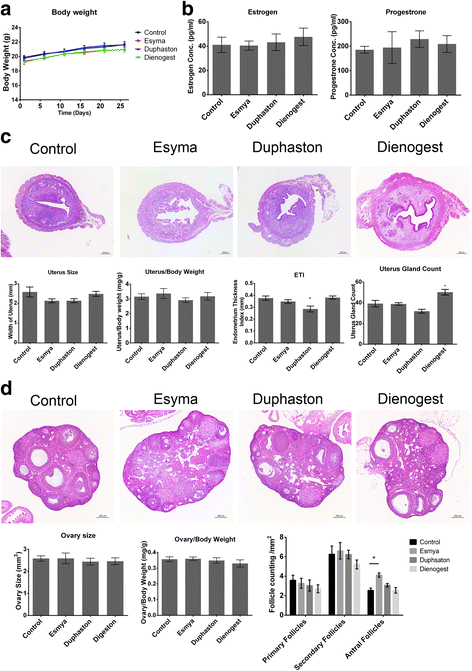
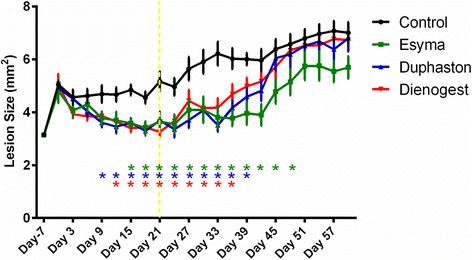
Results: After Esmya, Duphaston, Dienogest treatment, lesion size and weight were significantly decreased. Proliferation Pcna expression was significantly decreased in all groups, but proliferation cells were significantly decreased only in Duphaston group. Apoptosis Mapk1 expression and TUNEL-positive cells were significantly increased in Duphaston group. Adhesion Mmp2 and Itgavβ3 expression were significantly increased in Esmya group. Plau, Hif1α and Vegfa expression, peritoneal fluid PGE2 levels, and ERα and ERβ expression were not affected; while PR expression was significantly lower in all groups. Endometrial gland count in uterus was significantly increased in Dienogest group, ETI was significantly decreased in Duphaston group, and AFC were significantly increased in Esmya group. Upon treatment cessation, lesion growth rebound quickly in Dienogest and Duphaston groups, but slowly in Esmya group.
Conclusion: Esmya, Duphaston and Dienogest are effective anti-endometriosis drugs targeting proliferation, apoptosis and adhesion. Esmya, Duphaston and Dienogest are all well tolerable, although endometrial glandular hyperplasia was found in Dienogest, endometrial atrophy in Duphaston, follicle accumulation in Esmya.
Keywords: Endometrium; Progestin; Selective progesterone receptor modulator.
Conflict of interest statement
Ethics approval
All of the animal experiments were approved from the Animal Experimentation Ethics Committee, The Chinese University of Hong Kong.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–469. doi: 10.1016/S0002-9378(15)30003-X. - DOI
-
- Zeitoun K, Takayama K, Sasano H, Suzuki T, Moghrabi N, Andersson S, Johns A, Meng L, Putman M, Carr B, Bulun SE. Deficient 17beta-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17beta-estradiol. J Clin Endocrinol Metab. 1998;83:4474–4480. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

