Mature and progenitor endothelial cells perform angiogenesis also under protease inhibition: the amoeboid angiogenesis
- PMID: 29615071
- PMCID: PMC5883600
- DOI: 10.1186/s13046-018-0742-2
Mature and progenitor endothelial cells perform angiogenesis also under protease inhibition: the amoeboid angiogenesis
Abstract
Background: Controlling vascular growth is a challenging aim for the inhibition of tumor growth and metastasis. The amoeboid and mesenchymal types of invasiveness are two modes of migration interchangeable in cancer cells: the Rac-dependent mesenchymal migration requires the activity of proteases; the Rho-ROCK-dependent amoeboid motility is protease-independent and has never been described in endothelial cells.
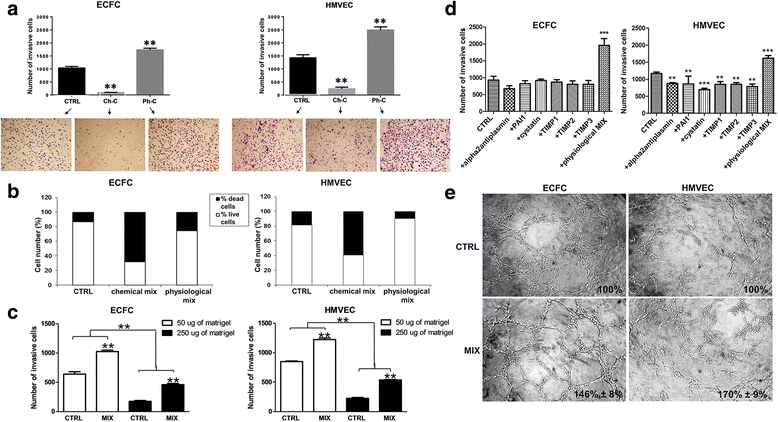
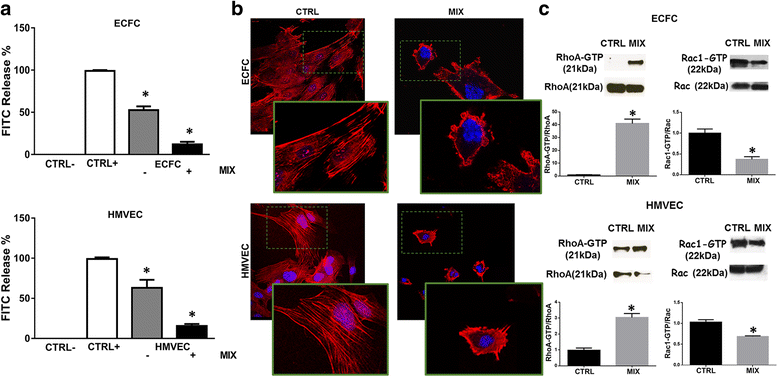
Methods: A cocktail of physiologic inhibitors (Ph-C) of serine-proteases, metallo-proteases and cysteine-proteases, mimicking the physiological environment that cells encounter during their migration within the angiogenesis sites was used to induce amoeboid style migration of Endothelial colony forming cells (ECFCs) and mature endothelial cells (ECs). To evaluate the mesenchymal-ameboid transition RhoA and Rac1 activation assays were performed along with immunofluorescence analysis of proteins involved in cytoskeleton organization. Cell invasion was studied in Boyden chambers and Matrigel plug assay for the in vivo angiogenesis.
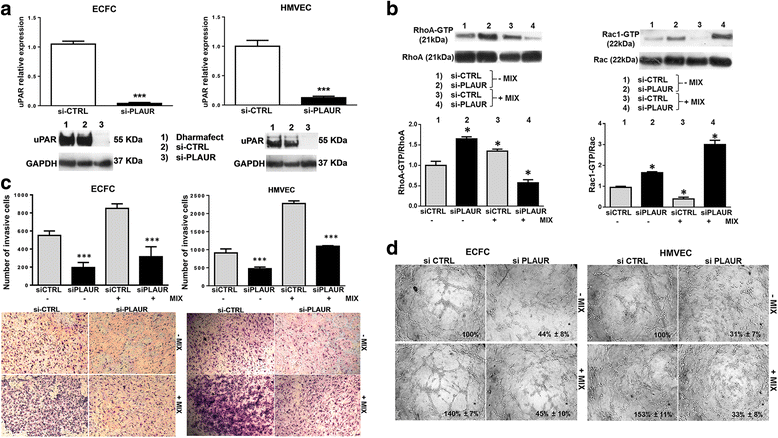
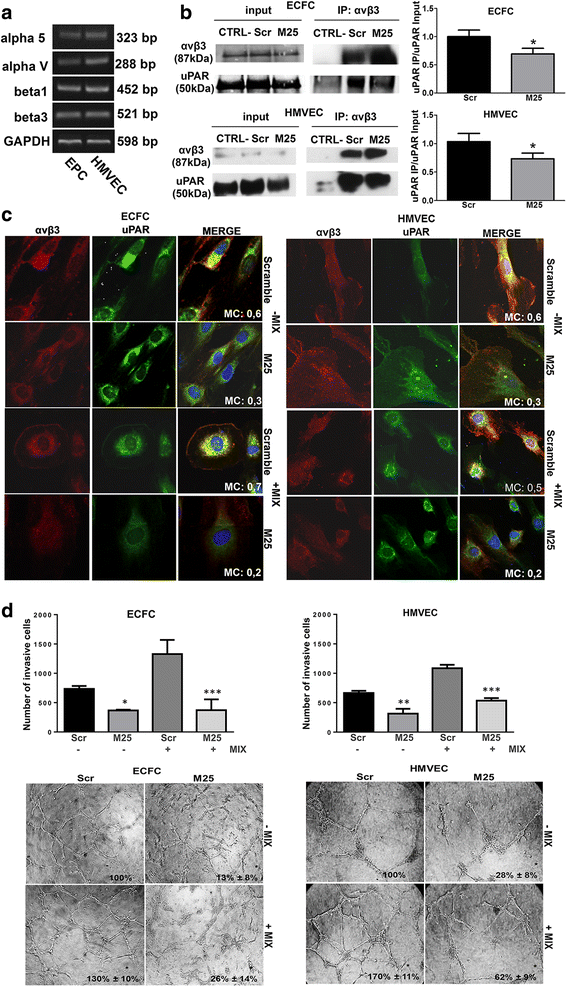
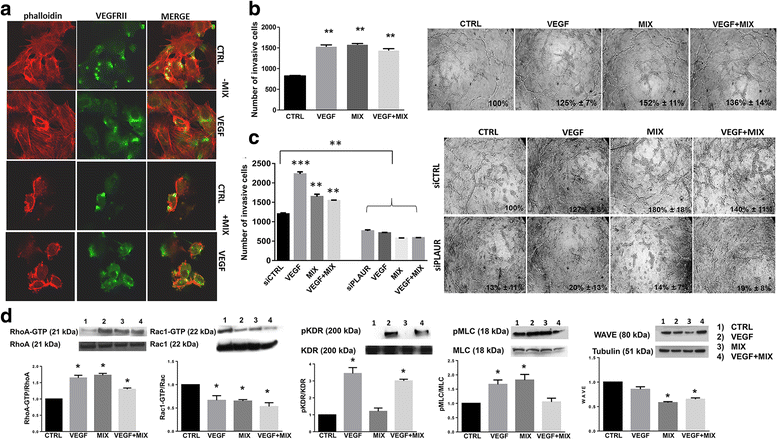
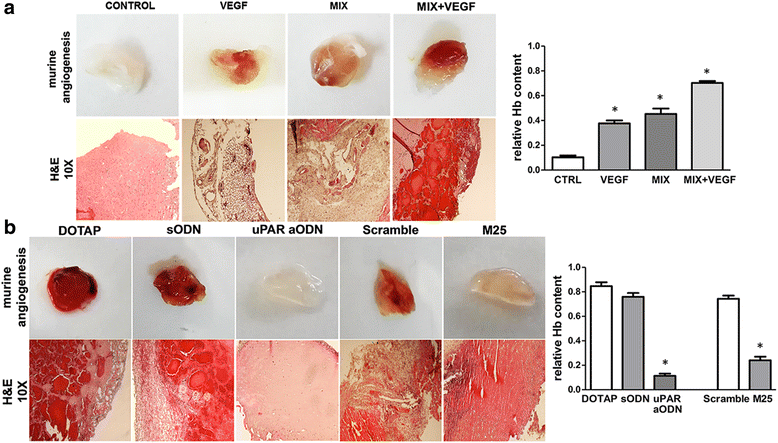
Results: In the present study we showed in both ECFCs and ECs, a decrease of activated Rac1 and an increase of activated RhoA upon shifting of cells to the amoeboid conditions. In presence of Ph-C inhibitors both cell lines acquired a round morphology and Matrigel invasion was greatly enhanced with respect to that observed in the absence of protease inhibition. We also observed that the urokinase-plasminogen-activator (uPAR) receptor silencing and uPAR-integrin uncoupling with the M25 peptide abolished both mesenchymal and amoeboid angiogenesis of ECFCs and ECs in vitro and in vivo, indicating a role of the uPAR-integrin-actin axis in the regulation of amoeboid angiogenesis. Furthermore, under amoeboid conditions endothelial cells seem to be indifferent to VEGF stimulation, which induces an amoeboid signaling pattern also in mesenchymal conditions.
Conclusion: Here we first provide a data set disclosing that endothelial cells can move and differentiate into vascular structures in vitro and in vivo also in the absence of proteases activity, performing a new type of neovascularization: the "amoeboid angiogenesis". uPAR is indispensable for ECs and ECFCs to perform an efficient amoeboid angiogenesis. Therefore, uPAR silencing or the block of its integrin-interaction, together with standard treatment against VEGF, could be a possible solution for angiogenesis inhibition.
Conflict of interest statement
Ethics approval and consent to participate
Endothelial Colony Forming Cells (ECFCs) were isolated from > 50 ml human umbilical cord blood (UCB) of healthy newborns after maternal informed consent and in compliance with Italian legislation. All procedures involving animals were performed in accordance with the ethical standards and according to the Declaration of Helsinki and to national guidelines, approved by the ethical committee of Animal Welfare Office of Italian Health Ministry and conformed to the legal mandates and Italian guidelines for the care and maintenance of laboratory animals.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Inhibition of MMPs supports amoeboid angiogenesis hampering VEGF-targeted therapies via MLC and ERK 1/2 signaling.J Transl Med. 2023 Feb 9;21(1):102. doi: 10.1186/s12967-023-03954-6. J Transl Med. 2023. PMID: 36759828 Free PMC article.
-
The receptor for urokinase-plasminogen activator (uPAR) controls plasticity of cancer cell movement in mesenchymal and amoeboid migration style.Oncotarget. 2014 Mar 30;5(6):1538-53. doi: 10.18632/oncotarget.1754. Oncotarget. 2014. PMID: 24681666 Free PMC article.
-
Long-Term Expansion in Platelet Lysate Increases Growth of Peripheral Blood-Derived Endothelial-Colony Forming Cells and Their Growth Factor-Induced Sprouting Capacity.PLoS One. 2015 Jun 15;10(6):e0129935. doi: 10.1371/journal.pone.0129935. eCollection 2015. PLoS One. 2015. PMID: 26076450 Free PMC article.
-
Endothelial Progenitor Cells in Sprouting Angiogenesis: Proteases Pave the Way.Curr Mol Med. 2015;15(7):606-20. doi: 10.2174/1566524015666150831131214. Curr Mol Med. 2015. PMID: 26321757 Review.
-
FilGAP and its close relatives: a mediator of Rho-Rac antagonism that regulates cell morphology and migration.Biochem J. 2013 Jul 1;453(1):17-25. doi: 10.1042/BJ20130290. Biochem J. 2013. PMID: 23763313 Review.
Cited by
-
Blood Flow Restriction Exercise Attenuates the Exercise-Induced Endothelial Progenitor Cell Response in Healthy, Young Men.Front Physiol. 2019 Apr 17;10:447. doi: 10.3389/fphys.2019.00447. eCollection 2019. Front Physiol. 2019. PMID: 31057427 Free PMC article.
-
Melanoma cells adopt features of both mesenchymal and amoeboid migration within confining channels.Sci Rep. 2021 Sep 7;11(1):17804. doi: 10.1038/s41598-021-97348-7. Sci Rep. 2021. PMID: 34493759 Free PMC article.
-
Activation of the Kinin B1 Receptor by Its Agonist Reduces Melanoma Metastasis by Playing a Dual Effect on Tumor Cells and Host Immune Response.Front Pharmacol. 2019 Sep 25;10:1106. doi: 10.3389/fphar.2019.01106. eCollection 2019. Front Pharmacol. 2019. PMID: 31607931 Free PMC article.
-
Therapeutic Strategies Targeting Urokinase and Its Receptor in Cancer.Cancers (Basel). 2022 Jan 19;14(3):498. doi: 10.3390/cancers14030498. Cancers (Basel). 2022. PMID: 35158766 Free PMC article. Review.
-
Multifunctional Heterometallic IrIII -AuI Probes as Promising Anticancer and Antiangiogenic Agents.Chemistry. 2021 Jul 7;27(38):9885-9897. doi: 10.1002/chem.202100707. Epub 2021 May 29. Chemistry. 2021. PMID: 33860585 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

