Improving the effectiveness of psychological interventions for depression and anxiety in the cardiac rehabilitation pathway using group-based metacognitive therapy (PATHWAY Group MCT): study protocol for a randomised controlled trial
- PMID: 29615092
- PMCID: PMC5883514
- DOI: 10.1186/s13063-018-2593-8
Improving the effectiveness of psychological interventions for depression and anxiety in the cardiac rehabilitation pathway using group-based metacognitive therapy (PATHWAY Group MCT): study protocol for a randomised controlled trial
Abstract
Background: Anxiety and depression are prevalent among cardiac rehabilitation patients but pharmacological and psychological treatments have limited effectiveness in this group. Furthermore, psychological interventions have not been systematically integrated into cardiac rehabilitation services despite being a strategic priority for the UK National Health Service. A promising new treatment, metacognitive therapy, may be well-suited to the needs of cardiac rehabilitation patients and has the potential to improve outcomes. It is based on the metacognitive model, which proposes that a thinking style dominated by rumination, worry and threat monitoring maintains emotional distress. Metacognitive therapy is highly effective at reducing this thinking style and alleviating anxiety and depression in mental health settings. This trial aims to evaluate the effectiveness and cost-effectiveness of group-based metacognitive therapy for cardiac rehabilitation patients with elevated anxiety and/or depressive symptoms.
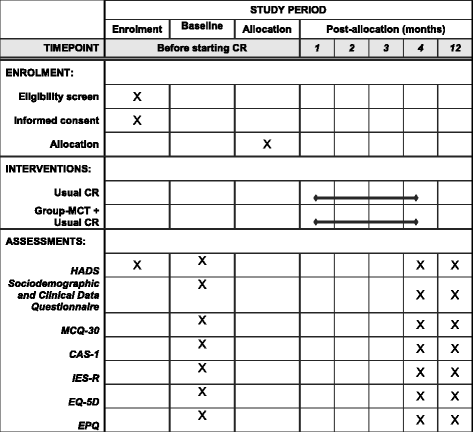
Methods/design: The PATHWAY Group-MCT trial is a multicentre, two-arm, single-blind, randomised controlled trial comparing the clinical- and cost-effectiveness of group-based metacognitive therapy plus usual cardiac rehabilitation to usual cardiac rehabilitation alone. Cardiac rehabilitation patients (target sample n = 332) with elevated anxiety and/or depressive symptoms will be recruited across five UK National Health Service Trusts. Participants randomised to the intervention arm will receive six weekly sessions of group-based metacognitive therapy delivered by either cardiac rehabilitation professionals or research nurses. The intervention and control groups will both be offered the usual cardiac rehabilitation programme within their Trust. The primary outcome is severity of anxiety and depressive symptoms at 4-month follow-up measured by the Hospital Anxiety and Depression Scale total score. Secondary outcomes are severity of anxiety/depression at 12-month follow-up, health-related quality of life, severity of post-traumatic stress symptoms and strength of metacognitive beliefs at 4- and 12-month follow-up. Qualitative interviews will help to develop an account of barriers and enablers to the effectiveness of the intervention.
Discussion: This trial will evaluate the effectiveness and cost-effectiveness of group-based metacognitive therapy in alleviating anxiety and depression in cardiac rehabilitation patients. The therapy, if effective, offers the potential to improve psychological wellbeing and quality of life in this large group of patients.
Trial registration: UK Clinical Trials Gateway, ISRCTN74643496 , Registered on 8 April 2015.
Keywords: Metacognitive therapy; anxiety; cardiac rehabilitation; depression; group therapy; heart disease; psychological intervention; rumination; worry.
Conflict of interest statement
Ethics approval and consent to participate
The trial received full ethical approval from Preston Research Ethics Committee on 6 March 2015: REC Reference 14/NW/0163. The following sites have approved the trial: University Hospital of South Manchester NHS Foundation Trust; Central Manchester University Hospitals NHS Foundation Trust; East Cheshire NHS Trust; Stockport NHS Foundation Trust, and Pennine Acute Hospitals NHS Trust. The trial has obtained an International Standard Randomised Controlled Trial Number (ISRCTN reference: ISRCTN74643496). Any modifications to the protocol will be submitted for further ethical approval and approved changes will be documented on the ISRCTN registry. The trial will be conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki, 1996; the principles of Good Clinical Practice, and the Department of Health Research Governance Framework for Health and Social Care, 2005. Written informed consent will be obtained from all participants in the trial. Copies of the consent forms will be kept in the trial site files and the patients’ medical notes. Participants will be free to withdraw from the study at any time without giving a reason. All the information collected during this trial will be confidential and held in accordance with NHS Data Protection guidelines and Good Clinical Practice guidelines. Confidentiality will only be breached if patients disclose to us information which may indicate that there is a risk of harm to themselves or others.
Consent for publication
Not applicable.
Competing interests
Professor Adrian Wells is the developer of metacognitive therapy and a co-director of the Metacognitive Therapy Institute.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- National Audit of Cardiac Rehabilitation . Annual Report. York: British Heart Foundation, University of York; 2012.
-
- NHS Scotland. Cardiac Rehabilitation in Scotland. 2013. http://www.isdscotland.org/Health-Topics/Heart-Disease/Publications/. Accessed 22 July 2017.
-
- NHS England. Increase Uptake of Cardiac Rehabilitation for People with Coronary Artery Disease and Following Acute Heart Failure. 2014. https://www.england.nhs.uk/wp-content/uploads/2014/02/pm-fs-3-10.pdf. Accessed 22 July 2017.
-
- Meijer A, Conradi HJ, Bos EH, Thombs BD, van Melle JP, de Jonge P. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: A meta-analysis of 25 years of research. Gen Hosp Psychiatry. 2011;33(3):203–216. doi: 10.1016/j.genhosppsych.2011.02.007. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

