Structural and Regulatory Changes in PBP4 Trigger Decreased β-Lactam Susceptibility in Enterococcus faecalis
- PMID: 29615500
- PMCID: PMC5885037
- DOI: 10.1128/mBio.00361-18
Structural and Regulatory Changes in PBP4 Trigger Decreased β-Lactam Susceptibility in Enterococcus faecalis
Abstract
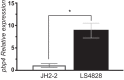
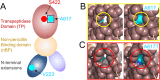
Enterococcus faecalis strains resistant to penicillin and ampicillin are rare and have been associated with increases in quantities of low-affinity penicillin-binding protein 4 (PBP4) or with amino acid substitutions in PBP4. We report an E. faecalis strain (LS4828) isolated from a prosthetic knee joint that was subjected to long-term exposure to aminopenicillins. Subsequent cultures yielded E. faecalis with MICs of penicillins and carbapenems higher than those for wild-type strain E. faecalis JH2-2. Sequence analysis of the pbp4 gene of LS4828 compared to that of JH2-2 revealed two point mutations with amino acid substitutions (V223I, A617T) and deletion of an adenine from the region upstream of the predicted pbp4 -35 promoter sequence (UP region). Purified PBP4 from LS4828 exhibited less affinity for Bocillin FL than did PBP4 from JH2-2, which was recapitulated by purified PBP4 containing only the A617T mutation. Differential scanning fluorimetry studies showed that the LS4828 and A617T variants are destabilized compared to wild-type PBP4. Further, reverse transcription-PCR indicated increased transcription of pbp4 in LS4828 and Western blot analysis with polyclonal PBP4 antibody revealed greater quantities of PBP4 in LS4828 than in JH2-2 lysates and membrane preparations. Placing the promoter regions from LS4828 or JH2-2 upstream of a green fluorescent protein reporter gene confirmed that the adenine deletion was associated with increased transcription. Together, these data suggest that the reduced susceptibility to β-lactam antibiotics observed in E. faecalis LS4828 results from a combination of both increased expression and remodeling of the active site, resulting in reduced affinity for penicillins and carbapenems.IMPORTANCEEnterococcus faecalis is an important cause of community-acquired and nosocomial infections and creates therapeutic dilemmas because of its frequent resistance to several classes of antibiotics. We report an E. faecalis strain with decreased ampicillin and imipenem susceptibility isolated after prolonged courses of aminopenicillin therapy for a prosthetic joint infection. Its reduced susceptibility is attributable to a combination of increased quantities of low-affinity PBP4 and an amino acid substitution in proximity to the active site that destabilizes the protein. Our findings provide a cautionary tale for clinicians who elect to "suppress" infections in prosthetic joints and offer novel insights into the interaction of β-lactam antibiotics with low-affinity PBP4. These insights will help inform future efforts to develop therapeutics capable of inhibiting clinical enterococcal strains.
Keywords: Enterococcus; antibiotic resistance; penicillin-binding proteins.
Copyright © 2018 Rice et al.
Figures




References
-
- Fernández-Hidalgo N, Almirante B, Gavaldà J, Gurgui M, Peña C, de Alarcón A, Ruiz J, Vilacosta I, Montejo M, Vallejo N, López-Medrano F, Plata A, López J, Hidalgo-Tenorio C, Gálvez J, Sáez C, Lomas JM, Falcone M, de la Torre J, Martínez-Lacasa X, Pahissa A. 2013. Ampicillin plus ceftriaxone is as effective as ampicillin plus gentamicin for treating Enterococcus faecalis infective endocarditis. Clin Infect Dis 56:1261–1268. doi: 10.1093/cid/cit052. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

