Towards an arthritis flare-responsive drug delivery system
- PMID: 29615615
- PMCID: PMC5882944
- DOI: 10.1038/s41467-018-03691-1
Towards an arthritis flare-responsive drug delivery system
Erratum in
-
Author Correction: Towards an arthritis flare-responsive drug delivery system.Nat Commun. 2018 May 11;9(1):1954. doi: 10.1038/s41467-018-04346-x. Nat Commun. 2018. PMID: 29752435 Free PMC article.
Abstract
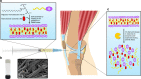
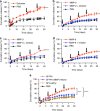
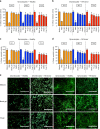
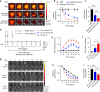
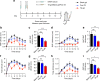
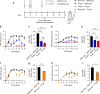
Local delivery of therapeutics for the treatment of inflammatory arthritis (IA) is limited by short intra-articular half-lives. Since IA severity often fluctuates over time, a local drug delivery method that titrates drug release to arthritis activity would represent an attractive paradigm in IA therapy. Here we report the development of a hydrogel platform that exhibits disassembly and drug release controlled by the concentration of enzymes expressed during arthritis flares. In vitro, hydrogel loaded with triamcinolone acetonide (TA) releases drug on-demand upon exposure to enzymes or synovial fluid from patients with rheumatoid arthritis. In arthritic mice, hydrogel loaded with a fluorescent dye demonstrates flare-dependent disassembly measured as loss of fluorescence. Moreover, a single dose of TA-loaded hydrogel but not the equivalent dose of locally injected free TA reduces arthritis activity in the injected paw. Together, our data suggest flare-responsive hydrogel as a promising next-generation drug delivery approach for the treatment of IA.
Conflict of interest statement
J.M.K. holds equity in Alivio Therapeutics, a company that has an option to license IP generated by J.M.K., and that may benefit financially if the IP is licensed and further validated. The interests of J.M.K. was reviewed and is subject to a management plan overseen by his institution in accordance with its conflict of interest policies. J.M.K., N.J., S.B., K.V.S., X.H., J.A., and P.K.V. have the following public patents based on this work: 1. Patent applicant: Brigham and Women’s Hospital, The City University of New York and inventors (name of inventors: J.M.K., P.K.V., George John, and Greg Cruikshank. Application number: PCT/US2009/057349. Status of application: Application undergoing pre-examination processing. Specific aspect of work covered: hydrogel). 2. Patent applicant: Brigham and Women’s Hospital and inventors (name of inventors: J.M.K., P.K.V., Nathaniel R. Campbell, Abdullah M. Syed, Sufeng Zhang, Omid C. Farokhzad, and Robert S. Langer. Application number: PCT/US2011/053075. Status of application: With receiving office. Specific aspect of the work covered in patent application: hydrogel). 3. Patent applicant: Brigham and Women’s Hospital and inventors (name of inventors: J.M.K., N.J., Nikken Wiradharma, and K.V.S. Application number: PCT/US2016/056070. Status of application: Published. Specific aspect of manuscript covered in the patent application: hydrogel). 4. Patent applicant: Brigham and Women’s Hospital and inventors (name of inventors: J.M.K., N.J., X.H., and S.B. Application number: PCT/US2017/031615. Status of application: Published. Specific aspect of manuscript covered in patent application: hydrogel). 5. Patent applicant: Brigham and Women’s Hospital and inventors (name of inventors: J.M.K., N.J., X.H., J.A., Britanny Laramee, and K.V.S. Application number: PCT/US2017/031614. Status of application: Published. Specific aspect of manuscript covered in patent application: hydrogel). J.M.K., N.J., and N.E.S. also have one unpublished patent based on the hydrogel work presented in this manuscript. A.O.A. is a current employee of Merck and Co. The remaining authors declare no competing interests.
Figures
Comment in
-
Drugs deliver themselves during flares.Nat Rev Rheumatol. 2018 Jun;14(6):321. doi: 10.1038/s41584-018-0014-8. Nat Rev Rheumatol. 2018. PMID: 29743628 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

