A powerful on line ABTS+-CE-DAD method to screen and quantify major antioxidants for quality control of Shuxuening Injection
- PMID: 29615669
- PMCID: PMC5883040
- DOI: 10.1038/s41598-018-23748-x
A powerful on line ABTS+-CE-DAD method to screen and quantify major antioxidants for quality control of Shuxuening Injection
Abstract
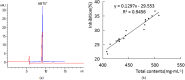
A novel method of on-line 2,2'-Azinobis-(3-ethylbenzthiazoline-6-sulphonate)-Capillary Electrophoresis-Diode Array Detector (on-line ABTS+-CE-DAD) was developed to screen the major antioxidants from complex herbal medicines. ABTS+, one of well-known oxygen free radicals was firstly integrated into the capillary. For simultaneously detecting and separating ABTS+ and chemical components of herb medicines, some conditions were optimized. The on-line ABTS+-CE-DAD method has successfully been used to screen the main antioxidants from Shuxuening injection (SI), an herbal medicines injection. Under the optimum conditions, nine ingredients of SI including clitorin, rutin, isoquercitrin, Quercetin-3-O-D-glucosyl]-(1-2)-L-rhamnoside, kaempferol-3-O-rutinoside, kaempferol-7-O-β-D-glucopyranoside, apigenin-7-O-Glucoside, quercetin-3-O-[2-O-(6-O-p-hydroxyl-E-coumaroyl)-D-glucosyl]-(1-2)-L-rhamnoside, 3-O-{2-O-[6-O-(p-hydroxyl-E-coumaroyl)-glucosyl]}-(1-2) rhamnosyl kaempfero were separated and identified as the major antioxidants. There is a linear relationship between the total amount of major antioxidants and total antioxidative activity of SI with a linear correlation coefficient of 0.9456. All the Relative standard deviations of recovery, precision and stability were below 7.5%. Based on these results, these nine ingredients could be selected as combinatorial markers to evaluate quality control of SI. It was concluded that on-line ABTS+-CE-DAD method was a simple, reliable and powerful tool to screen and quantify active ingredients for evaluating quality of herbal medicines.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
A novel reverse migration micellar electrokinetic chromatography method for in-capillary screening and quantifying of antioxidant components in Sanyetangzhiqing using 2,2'-Azinobis-(3-ethylbenzthiazoline-6-sulphonate) as oxidation-free radical.Electrophoresis. 2022 Jun;43(11):1148-1160. doi: 10.1002/elps.202100330. Epub 2022 Apr 20. Electrophoresis. 2022. PMID: 35287248
-
The in-capillary- 2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)-sweeping micellar electrokinetic chromatography - Diode array detector method for screening and quantifying trace natural antioxidants from Schisandra chinensis.J Chromatogr A. 2019 May 24;1593:147-155. doi: 10.1016/j.chroma.2019.01.050. Epub 2019 Jan 19. J Chromatogr A. 2019. PMID: 30685187
-
Multiple on-line HPLC coupled with biochemical detection methods to evaluate bioactive compounds in Danshen injection.Biomed Chromatogr. 2016 Nov;30(11):1854-1860. doi: 10.1002/bmc.3772. Epub 2016 Jul 12. Biomed Chromatogr. 2016. PMID: 27229445
-
Comparison of antioxidant activities of different parts from snow chrysanthemum (Coreopsis tinctoria Nutt.) and identification of their natural antioxidants using high performance liquid chromatography coupled with diode array detection and mass spectrometry and 2,2'-azinobis(3-ethylbenzthiazoline-sulfonic acid)diammonium salt-based assay.J Chromatogr A. 2016 Jan 8;1428:134-42. doi: 10.1016/j.chroma.2015.10.037. Epub 2015 Oct 19. J Chromatogr A. 2016. PMID: 26521095
-
In-capillary screening and quantifying the antioxidants of Yangxinshi Tablet using field-enhanced sample injection-micellar electrokinetic chromatography.J Sep Sci. 2023 Jul;46(13):e2300054. doi: 10.1002/jssc.202300054. Epub 2023 Apr 22. J Sep Sci. 2023. PMID: 37029534
Cited by
-
Recent Applications of Capillary Electrophoresis in the Determination of Active Compounds in Medicinal Plants and Pharmaceutical Formulations.Molecules. 2021 Jul 7;26(14):4141. doi: 10.3390/molecules26144141. Molecules. 2021. PMID: 34299418 Free PMC article. Review.
-
Emerging biotechnology applications in natural product and synthetic pharmaceutical analyses.Acta Pharm Sin B. 2022 Nov;12(11):4075-4097. doi: 10.1016/j.apsb.2022.08.025. Epub 2022 Sep 5. Acta Pharm Sin B. 2022. PMID: 36386468 Free PMC article. Review.
-
Clitorin ameliorates western diet-induced hepatic steatosis by regulating lipogenesis and fatty acid oxidation in vivo and in vitro.Sci Rep. 2022 Mar 9;12(1):4154. doi: 10.1038/s41598-022-07937-3. Sci Rep. 2022. PMID: 35264693 Free PMC article.
-
Prevention and treatment of osteoporosis with natural products: Regulatory mechanism based on cell ferroptosis.J Orthop Surg Res. 2023 Dec 11;18(1):951. doi: 10.1186/s13018-023-04448-3. J Orthop Surg Res. 2023. PMID: 38082321 Free PMC article. Review.
-
Ginkgo Flavonol Glycosides or Ginkgolides Tend to Differentially Protect Myocardial or Cerebral Ischemia-Reperfusion Injury via Regulation of TWEAK-Fn14 Signaling in Heart and Brain.Front Pharmacol. 2019 Jul 5;10:735. doi: 10.3389/fphar.2019.00735. eCollection 2019. Front Pharmacol. 2019. PMID: 31333457 Free PMC article.
References
-
- Cieslik E, et al. Contents of polyphenols in fruit and vegetables. Food Chem. 2006;94:135–142. doi: 10.1016/j.foodchem.2004.11.015. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

