Bepotastine besilate ophthalmic solution 1.5% for alleviating nasal symptoms in patients with allergic conjunctivitis
- PMID: 29615844
- PMCID: PMC5870655
- DOI: 10.2147/JAA.S160687
Bepotastine besilate ophthalmic solution 1.5% for alleviating nasal symptoms in patients with allergic conjunctivitis
Abstract
Background: Bepotastine besilate ophthalmic solution (BBOS) 1.5% is a topical antihistamine for the treatment of ocular itching associated with allergic conjunctivitis (AC). Allergic rhinitis and AC are common comorbid conditions. We explored the efficacy of BBOS 1.5% in alleviating nasal symptoms in an integrated analysis of two Phase III conjunctival allergen challenge (CAC) studies and a Phase IV environmental allergen study.
Methods: In the Phase III trials, a CAC was performed 15 minutes, 8 hours, and 16 hours following ocular instillation of BBOS 1.5% (n=78) or placebo (n=79), and subjects evaluated nasal symptoms. In the environmental study, subjects instilled BBOS 1.5% (n=123) or placebo (n=122) twice daily and nasal symptoms were evaluated over 2 weeks.
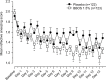
Results: In the Phase III trials, BBOS 1.5% had reduced CAC-induced nasal congestion and pruritus at 15 minutes and 8 hours postdosing and rhinorrhea and a non-ocular composite-symptom score (sum of nasal scores plus ear or palate pruritus) at all time points postdosing (all P≤0.01 vs placebo). In the Phase IV environmental study, BBOS 1.5% reduced sneezing and nasal pruritus over 2 weeks and median number of days to improvement of nasal pruritus and total nasal symptom score (sum for rhinorrhea, sneezing, nasal pruritus, and nasal congestion; P≤0.04 vs placebo). Additionally, investigator-reported improvement in overall ocular (pruritus, hyperemia, tearing) and nasal symptoms was greater with BBOS 1.5% vs placebo (P≤0.03).
Conclusion: Results of these exploratory analyses indicate that topical ocular BBOS 1.5% reduced nasal symptoms, supporting its use for alleviating rhinitis symptoms associated with AC.
Keywords: allergic rhinitis; antihistamine; bepotastine besilate; conjunctival allergen challenge; conjunctivitis; nasal symptoms.
Conflict of interest statement
Disclosure MEC and JIW are employees of Bausch … Lomb Incorporated. PJG is an employee of Ora. The authors report no other conflicts of interest in this work.
Figures

Similar articles
-
Time to onset and duration of action of the antihistamine bepotastine besilate ophthalmic solutions 1.0% and 1.5% in allergic conjunctivitis: a phase III, single-center, prospective, randomized, double-masked, placebo-controlled, conjunctival allergen challenge assessment in adults and children.Clin Ther. 2009 Sep;31(9):1908-21. doi: 10.1016/j.clinthera.2009.09.001. Clin Ther. 2009. PMID: 19843481 Clinical Trial.
-
Integrated phase III trials of bepotastine besilate ophthalmic solution 1.5% for ocular itching associated with allergic conjunctivitis.Allergy Asthma Proc. 2012 May-Jun;33(3):265-74. doi: 10.2500/aap.2012.33.3570. Allergy Asthma Proc. 2012. PMID: 22991696 Clinical Trial.
-
Prolonged effectiveness of bepotastine besilate ophthalmic solution for the treatment of ocular symptoms of allergic conjunctivitis.J Ocul Pharmacol Ther. 2011 Aug;27(4):385-93. doi: 10.1089/jop.2011.0005. Epub 2011 Jun 7. J Ocul Pharmacol Ther. 2011. PMID: 21649522 Clinical Trial.
-
Bepotastine besilate for the treatment of pruritus.Expert Opin Pharmacother. 2013 Dec;14(18):2553-69. doi: 10.1517/14656566.2013.849242. Epub 2013 Nov 5. Expert Opin Pharmacother. 2013. PMID: 24191914 Review.
-
Levocetirizine for the treatment of allergic rhinitis and chronic idiopathic urticaria in adults and children.Clin Ther. 2009 Aug;31(8):1664-87. doi: 10.1016/j.clinthera.2009.08.015. Clin Ther. 2009. PMID: 19808127 Review.
Cited by
-
Efficacy and Toxicity Evaluation of Bepotastine Besilate 1.5% Preservative-Free Eye Drops Vs Olopatadine Hydrochloride 0.2% Bak-Preserved Eye Drops in Patients with Allergic Conjunctivitis.Clin Ophthalmol. 2023 Nov 16;17:3477-3489. doi: 10.2147/OPTH.S431889. eCollection 2023. Clin Ophthalmol. 2023. PMID: 38026598 Free PMC article. Clinical Trial.
-
Inhibition of RAN attenuates influenza a virus replication and nucleoprotein nuclear export.Emerg Microbes Infect. 2024 Dec;13(1):2387910. doi: 10.1080/22221751.2024.2387910. Epub 2024 Aug 12. Emerg Microbes Infect. 2024. PMID: 39087696 Free PMC article.
-
Phase III trials examining the efficacy of cetirizine ophthalmic solution 0.24% compared to vehicle for the treatment of allergic conjunctivitis in the conjunctival allergen challenge model.Clin Ophthalmol. 2018 Dec 13;12:2617-2628. doi: 10.2147/OPTH.S185835. eCollection 2018. Clin Ophthalmol. 2018. PMID: 30587908 Free PMC article.
References
-
- Bielory L. Allergic conjunctivitis and the impact of allergic rhinitis. Curr Allergy Asthma Rep. 2010;10(2):122–134. - PubMed
-
- Hopper JL, Jenkins MA, Carlin JB, Giles GG. Increase in the self-reported prevalence of asthma and hay fever in adults over the last generation: a matched parent-offspring study. Aust J Public Health. 1995;19(2):120–124. - PubMed
-
- Blaiss MS. Allergic rhinoconjunctivitis: burden of disease. Allergy Asthma Proc. 2007;28(4):393–397. - PubMed
-
- Singh K, Axelrod S, Bielory L. The epidemiology of ocular and nasal allergy in the United States, 1988–1994. J Allergy Clin Immunol. 2010;126(4):778–783.e6. - PubMed
-
- Wallace DV, Dykewicz MS, Bernstein DI, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122(2 Suppl):S1–S84. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

