The Regulatory Mechanisms and Therapeutic Potential of MicroRNAs: From Chronic Pain to Morphine Tolerance
- PMID: 29615865
- PMCID: PMC5864932
- DOI: 10.3389/fnmol.2018.00080
The Regulatory Mechanisms and Therapeutic Potential of MicroRNAs: From Chronic Pain to Morphine Tolerance
Abstract
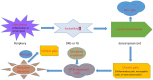
Chronic pain, including cancer-related pain, is a pain condition often caused by inflammation or dysfunctional nerves. Chronic pain treatment poses a significant health care challenge, where opioids especially morphine are widely used and patients often develop tolerance over time with aggravated pain. microRNA (miRNA) is known to play important roles in regulating gene expressions in the nervous system to affect neuronal network plasticity related to algogenesis and the developing of morphine tolerance. In this article, we reviewed studies conducted in rodent animal models investigating the mechanisms of miRNAs regulation in chronic pain with different phenotypes and morphine tolerance. In addition, the potential of targeting miRNAs for chronic pain and morphine tolerance treatment is also reviewed. Finally, we point out the directions of the future research in chronic pain and morphine tolerance.
Keywords: bone cancer pain; chronic pain; microRNA; microglia; morphine tolerance.
Figures


References
-
- Aldrich B. T., Frakes E. P., Kasuya J., Hammond D. L., Kitamoto T. (2009). Changes in expression of sensory organ-specific microRNAs in rat dorsal root ganglia in association with mechanical hypersensitivity induced by spinal nerve ligation. Neuroscience 164, 711–723. 10.1016/j.neuroscience.2009.08.033 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

