Advances in Glioblastoma Multiforme Treatment: New Models for Nanoparticle Therapy
- PMID: 29615917
- PMCID: PMC5868458
- DOI: 10.3389/fphys.2018.00170
Advances in Glioblastoma Multiforme Treatment: New Models for Nanoparticle Therapy
Abstract
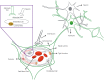
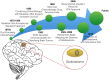
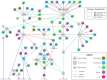
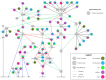
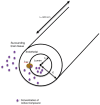
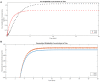
The most lethal form of brain cancer, glioblastoma multiforme, is characterized by rapid growth and invasion facilitated by cell migration and degradation of the extracellular matrix. Despite technological advances in surgery and radio-chemotherapy, glioblastoma remains largely resistant to treatment. New approaches to study glioblastoma and to design optimized therapies are greatly needed. One such approach harnesses computational modeling to support the design and delivery of glioblastoma treatment. In this paper, we critically summarize current glioblastoma therapy, with a focus on emerging nanomedicine and therapies that capitalize on cell-specific signaling in glioblastoma. We follow this summary by discussing computational modeling approaches focused on optimizing these emerging nanotherapeutics for brain cancer. We conclude by illustrating how mathematical analysis can be used to compare the delivery of a high potential anticancer molecule, delphinidin, in both free and nanoparticle loaded forms across the blood-brain barrier for glioblastoma.
Keywords: blood-brain barrier modeling; cytoscape; delphinidin; glioblastoma; nanoparticle.
Figures
References
-
- Amin F. U., Shah S. A., Badshah H., Khan M., Kim M. O. (2017). Anthocyanins encapsulated by PLGA@PEG nanoparticles potentially improved its free radical scavenging capabilities via p38/JNK pathway against Aβ1–42-induced oxidative stress. J. Nanobiotechnol. 15, 12. 10.1186/s12951-016-0227-4 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

