Muscle Atrophy Induced by Mechanical Unloading: Mechanisms and Potential Countermeasures
- PMID: 29615929
- PMCID: PMC5869217
- DOI: 10.3389/fphys.2018.00235
Muscle Atrophy Induced by Mechanical Unloading: Mechanisms and Potential Countermeasures
Abstract
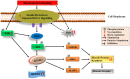
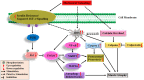
Prolonged periods of skeletal muscle inactivity or mechanical unloading (bed rest, hindlimb unloading, immobilization, spaceflight and reduced step) can result in a significant loss of musculoskeletal mass, size and strength which ultimately lead to muscle atrophy. With advancement in understanding of the molecular and cellular mechanisms involved in disuse skeletal muscle atrophy, several different signaling pathways have been studied to understand their regulatory role in this process. However, substantial gaps exist in our understanding of the regulatory mechanisms involved, as well as their functional significance. This review aims to update the current state of knowledge and the underlying cellular mechanisms related to skeletal muscle loss during a variety of unloading conditions, both in humans and animals. Recent advancements in understanding of cellular and molecular mechanisms, including IGF1-Akt-mTOR, MuRF1/MAFbx, FOXO, and potential triggers of disuse atrophy, such as calcium overload and ROS overproduction, as well as their role in skeletal muscle protein adaptation to disuse is emphasized. We have also elaborated potential therapeutic countermeasures that have shown promising results in preventing and restoring disuse-induced muscle loss. Finally, identified are the key challenges in this field as well as some future prospectives.
Keywords: disuse muscle atrophy; mechanical unloading; molecular and cellular pathways; protein degradation; protein synthesis; therapeutic countermeasures.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

