Hyper-Expression of PD-1 Is Associated with the Levels of Exhausted and Dysfunctional Phenotypes of Circulating CD161++TCR iVα7.2+ Mucosal-Associated Invariant T Cells in Chronic Hepatitis B Virus Infection
- PMID: 29616020
- PMCID: PMC5868455
- DOI: 10.3389/fimmu.2018.00472
Hyper-Expression of PD-1 Is Associated with the Levels of Exhausted and Dysfunctional Phenotypes of Circulating CD161++TCR iVα7.2+ Mucosal-Associated Invariant T Cells in Chronic Hepatitis B Virus Infection
Abstract
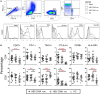
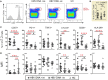
Mucosal-associated invariant T (MAIT) cells, defined as CD161++TCR iVα7.2+ T cells, play an important role in the innate defense against bacterial infections, and their functionality is impaired in chronic viral infections. Here, we investigated the frequency and functional role of MAIT cells in chronic hepatitis B virus (HBV) infection. The peripheral CD3+CD161++TCR iVα7.2+ MAIT cells in chronic HBV-infected patients and healthy controls were phenotypically characterized based on CD57, PD-1, TIM-3, and CTLA-4, as well as HLA-DR and CD38 expression. The frequency of MAIT cells was significantly decreased among chronic HBV-infected individuals as compared to controls. Expression of CD57, PD-1, CTLA-4, as well as HLA-DR and CD38 on MAIT cells was significantly elevated in chronic HBV-infected individuals relative to controls. The percentage of T cell receptor (TCR) iVα7.2+ CD161+ MAIT cells did not correlate with HBV viral load but inversely with HLA-DR on CD4+ T cells and MAIT cells and with CD57 on CD8+ T cells suggesting that decrease of MAIT cells may not be attributed to direct infection by HBV but driven by HBV-induced chronic immune activation. The percentage and expression levels of PD-1 as well as CTLA-4 on MAIT cells inversely correlated with plasma HBV-DNA levels, which may suggest either a role for MAIT cells in the control of HBV infection or the effect of HBV replication in the liver on MAIT cell phenotype. We report that decrease of TCR iVα7.2+ MAIT cells in the peripheral blood and their functions were seemingly impaired in chronic HBV-infected patients likely because of the increased expression of PD-1.
Keywords: CTLA-4; HBV infection; HLA-DR; PD-1; immune exhaustion; immunosenescence; mucosal-associated invariant T cells.
Figures
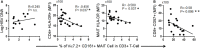



References
-
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (2012) 380(9859):2095–128.10.1016/S0140-6736(12)61728-0 - DOI - PMC - PubMed
-
- Vandepapeliere P, Lau GK, Leroux-Roels G, Horsmans Y, Gane E, Tawandee T, et al. Therapeutic vaccination of chronic hepatitis B patients with virus suppression by antiviral therapy: a randomized, controlled study of co-administration of HBsAg/AS02 candidate vaccine and lamivudine. Vaccine (2007) 25(51):8585–97.10.1016/j.vaccine.2007.09.072 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

