Recognition of Double-Stranded RNA and Regulation of Interferon Pathway by Toll-Like Receptor 10
- PMID: 29616030
- PMCID: PMC5865411
- DOI: 10.3389/fimmu.2018.00516
Recognition of Double-Stranded RNA and Regulation of Interferon Pathway by Toll-Like Receptor 10
Abstract
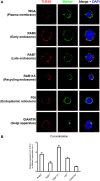
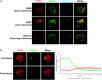
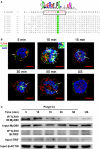
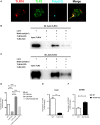
Toll-like receptor (TLR)-10 remains an orphan receptor without well-characterized ligands or functions. Here, we reveal that TLR10 is predominantly localized to endosomes and binds dsRNA in vitro at endosomal pH, suggesting that dsRNA is a ligand of TLR10. Recognition of dsRNA by TLR10 activates recruitment of myeloid differentiation primary response gene 88 for signal transduction and suppression of interferon regulatory factor-7 dependent type I IFN production. We also demonstrate crosstalk between TLR10 and TLR3, as they compete with each other for dsRNA binding. Our results suggest for the first time that dsRNA is a ligand for TLR10 and propose novel dual functions of TLR10 in regulating IFN signaling: first, recognition of dsRNA as a nucleotide-sensing receptor and second, sequestration of dsRNA from TLR3 to inhibit TLR3 signaling in response to dsRNA stimulation.
Keywords: IFN; TLR10; dsRNA; interferon regulatory factor; ligand sequestration; myeloid differentiation primary response gene 88; nucleotide-sensing receptor; toll-like receptor.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

