The Rice Rolled Fine Striped (RFS) CHD3/Mi-2 Chromatin Remodeling Factor Epigenetically Regulates Genes Involved in Oxidative Stress Responses During Leaf Development
- PMID: 29616070
- PMCID: PMC5870552
- DOI: 10.3389/fpls.2018.00364
The Rice Rolled Fine Striped (RFS) CHD3/Mi-2 Chromatin Remodeling Factor Epigenetically Regulates Genes Involved in Oxidative Stress Responses During Leaf Development
Abstract
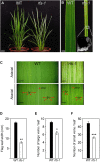
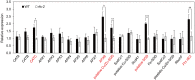
In rice (Oryza sativa), moderate leaf rolling increases photosynthetic competence and raises grain yield; therefore, this important agronomic trait has attracted much attention from plant biologists and breeders. However, the relevant molecular mechanism remains unclear. Here, we isolated and characterized Rolled Fine Striped (RFS), a key gene affecting rice leaf rolling, chloroplast development, and reactive oxygen species (ROS) scavenging. The rfs-1 gamma-ray allele and the rfs-2 T-DNA insertion allele of RFS failed to complement each other and their mutants had similar phenotypes, producing extremely incurved leaves due to defective development of vascular cells on the adaxial side. Map-based cloning showed that the rfs-1 mutant harbors a 9-bp deletion in a gene encoding a predicted CHD3/Mi-2 chromatin remodeling factor belonging to the SNF2-ATP-dependent chromatin remodeling family. RFS was expressed in various tissues and accumulated mainly in the vascular cells throughout leaf development. Furthermore, RFS deficiency resulted in a cell death phenotype that was caused by ROS accumulation in developing leaves. We found that expression of five ROS-scavenging genes [encoding catalase C, ascorbate peroxidase 8, a putative copper/zinc superoxide dismutase (SOD), a putative SOD, and peroxiredoxin IIE2] decreased in rfs-2 mutants. Western-blot and chromatin immunoprecipitation (ChIP) assays demonstrated that rfs-2 mutants have reduced H3K4me3 levels in ROS-related genes. Loss-of-function in RFS also led to multiple developmental defects, affecting pollen development, grain filling, and root development. Our results suggest that RFS is required for many aspects of plant development and its function is closely associated with epigenetic regulation of genes that modulate ROS homeostasis.
Keywords: Rolled Fine Striped; chloroplast biogenesis; chromatin remodeling factor; leaf variegation; narrow leaf; reactive oxygen species; rice (Oryza sativa).
Figures






References
-
- Agrawal G. K., Rakwal R., Jwa N.-S. (2001). Stress signaling molecules involved in defense and protein phosphatase 2A inhibitors modulate OsCATC expression in rice (Oryza sativa) seedlings. J. Plant Physiol. 158 1349–1355. 10.1078/0176-1617-00607 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

