The ATP-P2X7 Signaling Axis Is an Essential Sentinel for Intracellular Clostridium difficile Pathogen-Induced Inflammasome Activation
- PMID: 29616195
- PMCID: PMC5864904
- DOI: 10.3389/fcimb.2018.00084
The ATP-P2X7 Signaling Axis Is an Essential Sentinel for Intracellular Clostridium difficile Pathogen-Induced Inflammasome Activation
Erratum in
-
Corrigendum: The ATP-P2X7 Signaling Axis Is an Essential Sentinel for Intracellular Clostridium difficile Pathogen-Induced Inflammasome Activation.Front Cell Infect Microbiol. 2019 Jul 17;9:260. doi: 10.3389/fcimb.2019.00260. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31380303 Free PMC article.
Abstract
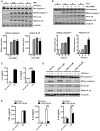
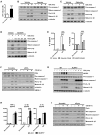
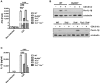
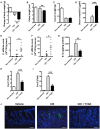
Clostridium difficile infection (CDI) is the leading cause of nosocomial infection in hospitalized patients receiving long-term antibiotic treatment. An excessive host inflammatory response is believed to be the major mechanism underlying the pathogenesis of C. difficile infection, and various proinflammatory cytokines such as IL-1β are detected in patients with C. difficile infection. IL-1β is known to be processed by caspase-1, a cysteine protease that is regulated by a protein complex called the inflammasome, which leads to a specialized form of cell death called pyroptosis. The function of inflammasome activation-induced pyroptosis is to clear or limit the spread of invading pathogens via infiltrated neutrophils. Here, we focused on inflammasome activation induced by intact C. difficile to re-evaluate the nature of inflammasome activation in CDI pathogenesis, which could provide information that leads to an alternative therapeutic strategy for the treatment of this condition in humans. First, we found that caspase-1-dependent IL-1β production was induced by C. difficile pathogens in macrophages and increased in a time-dependent manner. Moreover, intracellular toxigenic C. difficile was essential for ATP-P2X7 pathway of inflammasome activation and subsequent caspase-1-dependent pyroptotic cell death, leading to the loss of membrane integrity and release of intracellular contents such as LDH. Notably, we also observed that bacterial components such as surface layer proteins (SLPs) were released from pyroptotic cells. In addition, pro-IL-1β production was completely MyD88 and partially TLR2 dependent. Finally, to investigate the role of the caspase-1-dependent inflammasome in host defense, we found that colonic inflammasome activation was also induced by CDI and that caspase-1 inhibition by Ac-YVAD-CMK led to increased disease progression and C. difficile load. Taken together, the present results suggest that MyD88 and TLR2 are critical component in pro-IL-1β production and intracellular C. difficile following the ATP-P2X7 pathway of inflammasome activation and pyroptosis, which play important roles in host defense through the utilization of inflammation-mediated bacterial clearance mechanisms during C. difficile infection.
Keywords: ATP-P2X7 pathway; Clostridium difficile; MyD88; inflammasome activation; pyroptosis.
Figures


References
-
- Ausiello C. M., Cerquetti M., Fedele G., Spensieri F., Palazzo R., Nasso M., et al. (2006). Surface layer proteins from Clostridium difficile induce inflammatory and regulatory cytokines in human monocytes and dendritic cells. Microbes Infect. 8, 2640–2646. 10.1016/j.micinf.2006.07.009 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

