Plastid Transcript Editing across Dinoflagellate Lineages Shows Lineage-Specific Application but Conserved Trends
- PMID: 29617800
- PMCID: PMC5888634
- DOI: 10.1093/gbe/evy057
Plastid Transcript Editing across Dinoflagellate Lineages Shows Lineage-Specific Application but Conserved Trends
Abstract
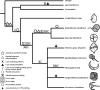
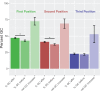
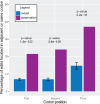
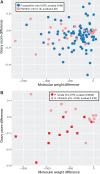
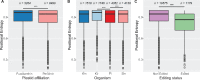
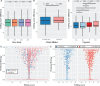
Dinoflagellates are a group of unicellular protists with immense ecological and evolutionary significance and cell biological diversity. Of the photosynthetic dinoflagellates, the majority possess a plastid containing the pigment peridinin, whereas some lineages have replaced this plastid by serial endosymbiosis with plastids of distinct evolutionary affiliations, including a fucoxanthin pigment-containing plastid of haptophyte origin. Previous studies have described the presence of widespread substitutional RNA editing in peridinin and fucoxanthin plastid genes. Because reports of this process have been limited to manual assessment of individual lineages, global trends concerning this RNA editing and its effect on the biological function of the plastid are largely unknown. Using novel bioinformatic methods, we examine the dynamics and evolution of RNA editing over a large multispecies data set of dinoflagellates, including novel sequence data from the peridinin dinoflagellate Pyrocystis lunula and the fucoxanthin dinoflagellate Karenia mikimotoi. We demonstrate that while most individual RNA editing events in dinoflagellate plastids are restricted to single species, global patterns, and functional consequences of editing are broadly conserved. We find that editing is biased toward specific codon positions and regions of genes, and generally corrects otherwise deleterious changes in the genome prior to translation, though this effect is more prevalent in peridinin than fucoxanthin lineages. Our results support a model for promiscuous editing application subsequently shaped by purifying selection, and suggest the presence of an underlying editing mechanism transferred from the peridinin-containing ancestor into fucoxanthin plastids postendosymbiosis, with remarkably conserved functional consequences in the new lineage.
Figures


References
-
- Bachvaroff TR, Concepcion GT, Rogers CR, Herman EM, Delwiche CF.. 2004. Dinoflagellate expressed sequence tag data indicate massive transfer of chloroplast genes to the nuclear genome. Protist 155(1):65–78. - PubMed
-
- Bailey TL, Elkan C.. 1994. Fitting a mixture model by expectation maximization to discover motifs in bipolymers. Proc Second Int Conf Intell Syst Mol Biol. 2:28–36. - PubMed
-
- Bailey TL, Gribskov M.. 1998. Combining evidence using p-values: application to sequence homology searches. Bioinformatics 14(1):48–54. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

