Weaning disrupts intestinal antioxidant status, impairs intestinal barrier and mitochondrial function, and triggers mitophagy in piglets
- PMID: 29617867
- PMCID: PMC6093500
- DOI: 10.1093/jas/skx062
Weaning disrupts intestinal antioxidant status, impairs intestinal barrier and mitochondrial function, and triggers mitophagy in piglets
Abstract
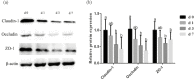
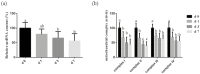
In the present study, we investigated the influence of weaning on antioxidant status, intestinal integrity, mitochondrial function, and the mitophagy level in piglets (weaned at 21 d) during the 1 wk after weaning. The redox status was measured by antioxidant enzymes activities, related genes expression, and malondialdehyde (MDA) content in jejunum. The intestinal barrier function was assessed by the Ussing chamber and expression of tight junction proteins in the jejunum. The function of intestine mitochondria was measured by mitochondrial DNA (mtDNA) content and activities of mitochondria oxidative phosphorylation complexes. The levels of light chain 3-1 (LC3-I), light chain 3-II (LC3-II), PTEN-induced putative kinase 1 (PINK1), and Parkin were determined to investigate whether mitophagy is involved in the weaning process. The results showed that, as compared with the preweaning phase (d 0), weaning suppressed (P < 0.05) the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) on d 3 and d 7 postweaning, decreased (P < 0.05) the expression of copper and zinc superoxide dismutase (Cu/Zn-SOD), manganese-containing superoxide dismutase (Mn-SOD) on d 3 postweaning, declined (P < 0.05) the level of glutathione peroxidase 1 (GPX-1) and glutathione peroxidase 4 (GPX-4) on d 3 and d 7 postweaning, and increased (P < 0.05) MDA content in jejunum on d 3 and d 7 postweaning. The jejunal transepithelial electrical resistance and levels of occludin, claudin-1, and zonula occludens-1 on d 3 and d 7 postweaning were reduced (P < 0.05), and paracellular flux of fluorescein isothiocyanatedextran (4 kDa) on d 3 and d 7 postweaning was increased (P < 0.05). Weaning induced mitochondrial dysfunction, as demonstrated by decreased (P < 0.05) content of mtDNA on d 3 and d 7 postweaning and declined (P < 0.05) activities of mitochondria complexes (I, II, III, IV) in jejunum on d 1, d 3, and d 7 postweaning. Weaning led to an increased (P < 0.05) expression level of mitophagy-related proteins, PINK1 and Parkin, in the intestinal mitochondria, as well as an enhancement (P < 0.05) of the ratio of LC3-II to LC3-I content in the jejunal mucosa on d 1, d 3, and d 7 postweaning. These results suggest that weaning disrupted intestinal oxidative balance, and this imbalance may impair intestinal barrier and mitochondrial function and trigger mitophagy in piglets.
Figures

References
-
- Afolayan A. J., Eis A., Alexander M., Michalkiewicz T., Teng R. J., Lakshminrusimha S., and Konduri G. G.. 2016. Decreased endothelial nitric oxide synthase expression and function contribute to impaired mitochondrial biogenesis and oxidative stress in fetal lambs with persistent pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 310:L40–L49. doi:10.1152/ajplung.00392.2014 - PMC - PubMed
-
- Assimakopoulos S. F., Vagianos C. E., Patsoukis N., Georgiou C., Nikolopoulou V., and Scopa C. D.. 2004. Evidence for intestinal oxidative stress in obstructive jaundice-induced gut barrier dysfunction in rats. Acta Physiol. Scand. 180:177–185. doi:10.1046/j.0001-6772.2003.01229.x - PubMed
-
- Bhat A. H., Dar K. B., Anees S., Zargar M. A., Masood A., Sofi M. A., and Ganie S. A.. 2015. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 74:101–110. doi:10.1016/j.biopha.2015.07.025 - PubMed
-
- Blachier F., Boutry C., Bos C., and Tome D.. 2009. Metabolism and functions ofl-glutamate in the epithelial cells of the small and large intestines. Am. J. Clin. Nutr. 90:814S–821S. doi:10.3945/ajcn.2009.27462S - PubMed
-
- Bomba L., Minuti A., Moisa S. J., Trevisi E., Eufemi E., Lizier M., Chegdani F., Lucchini F., Rzepus M., Prandini A.,. et al. 2014. Gut response induced by weaning in piglet features marked changes in immune and inflammatory response. Funct. Integr. Genomics 14:657–671. doi:10.1007/s10142-014-0396-x - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

