Gepotidacin for the Treatment of Uncomplicated Urogenital Gonorrhea: A Phase 2, Randomized, Dose-Ranging, Single-Oral Dose Evaluation
- PMID: 29617982
- PMCID: PMC6070052
- DOI: 10.1093/cid/ciy145
Gepotidacin for the Treatment of Uncomplicated Urogenital Gonorrhea: A Phase 2, Randomized, Dose-Ranging, Single-Oral Dose Evaluation
Abstract
Background: In this phase 2 study, we evaluated the efficacy and safety of oral gepotidacin, a novel triazaacenaphthylene bacterial type II topoisomerase inhibitor, for the treatment of uncomplicated urogenital gonorrhea.
Methods: Adult participants with suspected urogenital gonorrhea were enrolled and completed baseline (day 1) and test-of-cure (days 4-8) visits. Pretreatment and posttreatment urogenital swabs were collected for Neisseria gonorrhoeae (NG) culture and susceptibility testing. Pharyngeal and rectal swab specimens were collected if there were known exposures. Participants were stratified by gender and randomized 1:1 to receive a 1500-mg or 3000-mg single oral dose of gepotidacin.
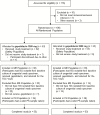
Results: The microbiologically evaluable population consisted of 69 participants, with NG isolated from 69 (100%) urogenital, 2 (3%) pharyngeal, and 3 (4%) rectal specimens. Microbiological eradication of NG was achieved by 97%, 95%, and 96% of participants (lower 1-sided exact 95% confidence interval bound, 85.1%, 84.7%, and 89.1%, respectively) for the 1500-mg, 3000-mg, and combined dose groups, respectively. Microbiological cure was achieved in 66/69 (96%) urogenital infections. All 3 failures were NG isolates that demonstrated the highest observed gepotidacin minimum inhibitory concentration of 1 µg/mL and a common gene mutation. At the pharyngeal and rectal sites, 1/2 and 3/3 NG isolates, respectively, demonstrated microbiological cure. There were no treatment-limiting adverse events for either dose.
Conclusions: This study demonstrated that single, oral doses of gepotidacin were ≥95% effective for bacterial eradication of NG in adult participants with uncomplicated urogenital gonorrhea.
Clinical trials registration: NCT02294682.
Figures

References
-
- World Health Organization. WHO guidelines for the treatment of Neisseria gonorrhoeae. 2016. Available at: http://apps.who.int/iris/bitstream/10665/246114/1/9789241549691-eng.pdf?.... Accessed 21 March 2017. - PubMed
-
- Centers for Disease Control and Prevention. Sexually transmitted diseases surveillance 2016. Atlanta, GA: CDC, 2017. Available at: https://www.cdc.gov/std/stats16/CDC_2016_STDS_Report-for508WebSep21_2017.... Accessed 12 October 2017.
-
- European Centre for Disease Prevention and Control. Annual epidemiological report 2016–gonorrhea. Stockholm, Sweden: ECDC, 2016. Available at: https://ecdc.europa.eu/sites/portal/files/documents/Gonorrhoea%20AER_0.pdf. Accessed 25 July 2017.
-
- Hook EW, Handsfield HH. Gonococcal infections in the adult. In: Holmes KK, Sparling PF, Stamm WE. et al. eds. Sexually transmitted diseases. 4th ed New York: McGraw-Hill, 2008:627–45.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

