Behavioral Variability and Phenotypic Diversity in Bacterial Chemotaxis
- PMID: 29618219
- PMCID: PMC5989721
- DOI: 10.1146/annurev-biophys-062215-010954
Behavioral Variability and Phenotypic Diversity in Bacterial Chemotaxis
Abstract
Living cells detect and process external signals using signaling pathways that are affected by random fluctuations. These variations cause the behavior of individual cells to fluctuate over time (behavioral variability) and generate phenotypic differences between genetically identical individuals (phenotypic diversity). These two noise sources reduce our ability to predict biological behavior because they diversify cellular responses to identical signals. Here, we review recent experimental and theoretical advances in understanding the mechanistic origin and functional consequences of such variation in Escherichia coli chemotaxis-a well-understood model of signal transduction and behavior. After briefly summarizing the architecture and logic of the chemotaxis system, we discuss determinants of behavior and chemotactic performance of individual cells. Then, we review how cell-to-cell differences in protein abundance map onto differences in individual chemotactic abilities and how phenotypic variability affects the performance of the population. We conclude with open questions to be addressed by future research.
Keywords: adaptation; chemical sensing; fluctuations; navigation; signal transduction; single-cell behavior.
Figures

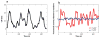
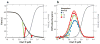
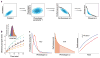
References
-
- Ackermann M. A functional perspective on phenotypic heterogeneity in microorganisms. Nat Rev Microbiol. 2015;13:497–508. - PubMed
-
- Adler J. Chemotaxis in bacteria. Science. 1966;153:708–16. - PubMed
-
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973;74:77–91. - PubMed
-
- Alon U, Surette MG, Barkai N, Leibler S. Robustness in bacterial chemotaxis. Nature. 1999;397:168–71. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

