Postnatal Zika virus infection is associated with persistent abnormalities in brain structure, function, and behavior in infant macaques
- PMID: 29618564
- PMCID: PMC6186170
- DOI: 10.1126/scitranslmed.aao6975
Postnatal Zika virus infection is associated with persistent abnormalities in brain structure, function, and behavior in infant macaques
Abstract
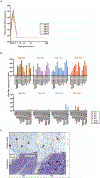
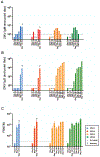
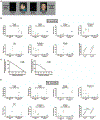
The Zika virus (ZIKV) epidemic is associated with fetal brain lesions and other serious birth defects classified as congenital ZIKV syndrome. Postnatal ZIKV infection in infants and children has been reported; however, data on brain anatomy, function, and behavioral outcomes following infection are absent. We show that postnatal ZIKV infection of infant rhesus macaques (RMs) results in persistent structural and functional alterations of the central nervous system compared to age-matched controls. We demonstrate ZIKV lymphoid tropism and neurotropism in infant RMs and histopathologic abnormalities in the peripheral and central nervous systems including inflammatory infiltrates, astrogliosis, and Wallerian degeneration. Structural and resting-state functional magnetic resonance imaging (MRI/rs-fMRI) show persistent enlargement of lateral ventricles, maturational changes in specific brain regions, and altered functional connectivity (FC) between brain areas involved in emotional behavior and arousal functions, including weakened amygdala-hippocampal connectivity in two of two ZIKV-infected infant RMs several months after clearance of ZIKV RNA from peripheral blood. ZIKV infection also results in distinct alterations in the species-typical emotional reactivity to acute stress, which were predicted by the weak amygdala-hippocampal FC. We demonstrate that postnatal ZIKV infection of infants in this model affects neurodevelopment, suggesting that long-term clinical monitoring of pediatric cases is warranted.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures




Similar articles
-
Long-term alterations in brain and behavior after postnatal Zika virus infection in infant macaques.Nat Commun. 2020 May 21;11(1):2534. doi: 10.1038/s41467-020-16320-7. Nat Commun. 2020. PMID: 32439858 Free PMC article.
-
Association Between Neonatal Neuroimaging and Clinical Outcomes in Zika-Exposed Infants From Rio de Janeiro, Brazil.JAMA Netw Open. 2019 Jul 3;2(7):e198124. doi: 10.1001/jamanetworkopen.2019.8124. JAMA Netw Open. 2019. PMID: 31365112 Free PMC article.
-
Sequential Neuroimaging of the Fetus and Newborn With In Utero Zika Virus Exposure.JAMA Pediatr. 2019 Jan 1;173(1):52-59. doi: 10.1001/jamapediatrics.2018.4138. JAMA Pediatr. 2019. PMID: 30476967 Free PMC article. Clinical Trial.
-
Zika infection and the development of neurological defects.Cell Microbiol. 2017 Jun;19(6). doi: 10.1111/cmi.12744. Epub 2017 May 3. Cell Microbiol. 2017. PMID: 28370966 Review.
-
Motor Abnormalities and Epilepsy in Infants and Children With Evidence of Congenital Zika Virus Infection.Pediatrics. 2018 Feb;141(Suppl 2):S167-S179. doi: 10.1542/peds.2017-2038F. Epub 2018 Feb 1. Pediatrics. 2018. PMID: 29437050 Review.
Cited by
-
Immune Evasion Strategies Used by Zika Virus to Infect the Fetal Eye and Brain.Viral Immunol. 2020 Jan/Feb;33(1):22-37. doi: 10.1089/vim.2019.0082. Epub 2019 Nov 5. Viral Immunol. 2020. PMID: 31687902 Free PMC article. Review.
-
Nonhuman Primate Models of Zika Virus Infection and Disease during Pregnancy.Viruses. 2021 Oct 16;13(10):2088. doi: 10.3390/v13102088. Viruses. 2021. PMID: 34696518 Free PMC article. Review.
-
Monodelphis domestica as a Fetal Intra-Cerebral Inoculation Model for Zika Virus Pathogenesis.Pathogens. 2023 May 19;12(5):733. doi: 10.3390/pathogens12050733. Pathogens. 2023. PMID: 37242404 Free PMC article.
-
A Rat Model of Prenatal Zika Virus Infection and Associated Long-Term Outcomes.Viruses. 2021 Nov 18;13(11):2298. doi: 10.3390/v13112298. Viruses. 2021. PMID: 34835104 Free PMC article.
-
Pre-existing Immunity to Japanese Encephalitis Virus Alters CD4 T Cell Responses to Zika Virus Inactivated Vaccine.Front Immunol. 2021 Feb 24;12:640190. doi: 10.3389/fimmu.2021.640190. eCollection 2021. Front Immunol. 2021. PMID: 33717194 Free PMC article. Clinical Trial.
References
-
- Brasil P, Pereira JP Jr., Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai UA, Salles TS, Zin AA, Horovitz D, Daltro P, Boechat M, Raja Gabaglia C, Carvalho de Sequeira P, Pilotto JH, Medialdea-Carrera R, Cotrim da Cunha D, Abreu de Carvalho LM, Pone M, Machado Siqueira A, Calvet GA, Rodrigues Baiao AE, Neves ES, Nassar de Carvalho PR, Hasue RH, Marschik PB, Einspieler C, Janzen C, Cherry JD, Bispo de Filippis AM, Nielsen-Saines K, Zika Virus Infection in Pregnant Women in Rio de Janeiro. N Engl J Med 375, 2321–2334 (2016). - PMC - PubMed
-
- Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, Vizjak A, Pizem J, Petrovec M, Avsic Zupanc T, Zika Virus Associated with Microcephaly. N Engl J Med 374, 951–958 (2016). - PubMed
-
- Dick GW, Kitchen SF, Haddow AJ, Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 46, 509–520 (1952). - PubMed
-
- Smithburn KC, Neutralizing antibodies against certain recently isolated viruses in the sera of human beings residing in East Africa. J Immunol 69, 223–234 (1952). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

