Wingless Signaling: A Genetic Journey from Morphogenesis to Metastasis
- PMID: 29618590
- PMCID: PMC5887133
- DOI: 10.1534/genetics.117.300157
Wingless Signaling: A Genetic Journey from Morphogenesis to Metastasis
Abstract
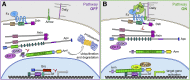
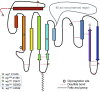
This FlyBook chapter summarizes the history and the current state of our understanding of the Wingless signaling pathway. Wingless, the fly homolog of the mammalian Wnt oncoproteins, plays a central role in pattern generation during development. Much of what we know about the pathway was learned from genetic and molecular experiments in Drosophila melanogaster, and the core pathway works the same way in vertebrates. Like most growth factor pathways, extracellular Wingless/Wnt binds to a cell surface complex to transduce signal across the plasma membrane, triggering a series of intracellular events that lead to transcriptional changes in the nucleus. Unlike most growth factor pathways, the intracellular events regulate the protein stability of a key effector molecule, in this case Armadillo/β-catenin. A number of mysteries remain about how the "destruction complex" destabilizes β-catenin and how this process is inactivated by the ligand-bound receptor complex, so this review of the field can only serve as a snapshot of the work in progress.
Keywords: FlyBook; Wingless; Wnt; beta-catenin; signal transduction.
Copyright © 2018 by the Genetics Society of America.
Figures


References
-
- Adler P. N., 1992. The genetic control of tissue polarity in Drosophila. Bioessays 14: 735–741. - PubMed
-
- Ahmed Y., Hayashi S., Levine A., Wieschaus E., 1998. Regulation of Armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell 93: 1171–1182. - PubMed
-
- Ahmed Y., Nouri A., Wieschaus E., 2002. Drosophila Apc1 and Apc2 regulate Wingless transduction throughout development. Development 129: 1751–1762. - PubMed
-
- Akong K., Grevengoed E. E., Price M. H., McCartney B. M., Hayden M. A., et al. , 2002. Drosophila APC2 and APC1 play overlapping roles in Wingless signaling in the embryo and imaginal discs. Dev. Biol. 250: 91–100. - PubMed
-
- Alexandre C., Lecourtois M., Vincent J., 1999. Wingless and Hedgehog pattern Drosophila denticle belts by regulating the production of short-range signals. Development 126: 5689–5698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

