Regulation of Vascular Calcification by Growth Hormone-Releasing Hormone and Its Agonists
- PMID: 29618597
- PMCID: PMC5948169
- DOI: 10.1161/CIRCRESAHA.117.312418
Regulation of Vascular Calcification by Growth Hormone-Releasing Hormone and Its Agonists
Abstract
Rationale: Vascular calcification (VC) is a marker of the severity of atherosclerotic disease. Hormones play important roles in regulating calcification; estrogen and parathyroid hormones exert opposing effects, the former alleviating VC and the latter exacerbating it. To date no treatment strategies have been developed to regulate clinical VC.
Objective: The objective of this study was to investigate the effect of growth hormone-releasing hormone (GHRH) and its agonist (GHRH-A) on the blocking of VC in a mouse model.
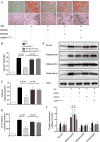
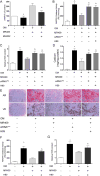
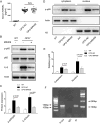
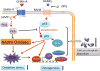
Methods and results: Young adult osteoprotegerin-deficient mice were given daily subcutaneous injections of GHRH-A (MR409) for 4 weeks. Significant reductions in calcification of the aortas of MR409-treated mice were paralleled by markedly lower alkaline phosphatase activity and a dramatic reduction in the expression of transcription factors, including the osteogenic marker gene Runx2 and its downstream factors, osteonectin and osteocalcin. The mechanism of action of GHRH-A was dissected in smooth muscle cells isolated from human and mouse aortas. Calcification of smooth muscle cells induced by osteogenic medium was inhibited in the presence of GHRH or MR409, as evidenced by reduced alkaline phosphatase activity and Runx2 expression. Inhibition of calcification by MR409 was partially reversed by MIA602, a GHRH antagonist, or a GHRH receptor-selective small interfering RNA. Treatment with MR409 induced elevated cytosolic cAMP and its target, protein kinase A which in turn blocked nicotinamide adenine dinucleotide phosphate oxidase activity and reduced production of reactive oxygen species, thus blocking the phosphorylation of nuclear factor κB (p65), a key intermediate in the ligand of receptor activator for nuclear factor-κ B-Runx2/alkaline phosphatase osteogenesis program. A protein kinase A-selective small interfering RNA or the chemical inhibitor H89 abolished these beneficial effects of MR409.
Conclusions: GHRH-A controls osteogenesis in smooth muscle cells by targeting cross talk between protein kinase A and nuclear factor κB (p65) and through the suppression of reactive oxygen species production that induces the Runx2 gene and alkaline phosphatase. Inflammation-mediated osteogenesis is thereby blocked. GHRH-A may represent a new pharmacological strategy to regulate VC.
Keywords: alkaline phosphatase; growth hormone-releasing hormone; myocytes, smooth muscle; osteogenesis; reactive oxygen species.
© 2018 American Heart Association, Inc.
Figures




References
-
- Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–18. - PubMed
-
- Abedin M, Tintut Y, Demer LL. Vascular calcification: Mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. - PubMed
-
- Huang H, Virmani R, Younis H, Burke AP, Kamm RD, Lee RT. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation. 2001;103:1051–1056. - PubMed
-
- Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

