Molecular structural diversity of mitochondrial cardiolipins
- PMID: 29618609
- PMCID: PMC5910844
- DOI: 10.1073/pnas.1719407115
Molecular structural diversity of mitochondrial cardiolipins
Abstract
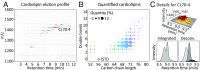
Current strategies used to quantitatively describe the biological diversity of lipids by mass spectrometry are often limited in assessing the exact structural variability of individual molecular species in detail. A major challenge is represented by the extensive isobaric overlap present among lipids, hampering their accurate identification. This is especially true for cardiolipins, a mitochondria-specific class of phospholipids, which are functionally involved in many cellular functions, including energy metabolism, cristae structure, and apoptosis. Substituted with four fatty acyl side chains, cardiolipins offer a particularly high potential to achieve complex mixtures of molecular species. Here, we demonstrate how systematically generated high-performance liquid chromatography-mass spectral data can be utilized in a mathematical structural modeling approach, to comprehensively analyze and characterize the molecular diversity of mitochondrial cardiolipin compositions in cell culture and disease models, cardiolipin modulation experiments, and a broad variety of frequently studied model organisms.
Keywords: cardiolipin; lipids; mass spectrometry; mathematical modeling; mitochondria.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Holthuis JCM, Menon AK. Lipid landscapes and pipelines in membrane homeostasis. Nature. 2014;510:48–57. - PubMed
-
- Zhang M, Mileykovskaya E, Dowhan W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J Biol Chem. 2002;277:43553–43556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

