Advanced maturation of human cardiac tissue grown from pluripotent stem cells
- PMID: 29618819
- PMCID: PMC5895513
- DOI: 10.1038/s41586-018-0016-3
Advanced maturation of human cardiac tissue grown from pluripotent stem cells
Erratum in
-
Author Correction: Advanced maturation of human cardiac tissue grown from pluripotent stem cells.Nature. 2019 Aug;572(7769):E16-E17. doi: 10.1038/s41586-019-1415-9. Nature. 2019. PMID: 31363231
Abstract
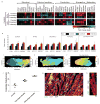
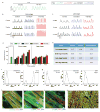
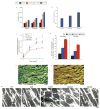
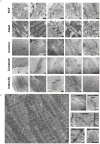
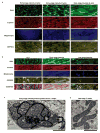
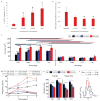
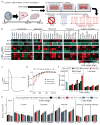
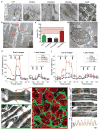
Cardiac tissues generated from human induced pluripotent stem cells (iPSCs) can serve as platforms for patient-specific studies of physiology and disease1-6. However, the predictive power of these models is presently limited by the immature state of the cells1, 2, 5, 6. Here we show that this fundamental limitation can be overcome if cardiac tissues are formed from early-stage iPSC-derived cardiomyocytes soon after the initiation of spontaneous contractions and are subjected to physical conditioning with increasing intensity over time. After only four weeks of culture, for all iPSC lines studied, such tissues displayed adult-like gene expression profiles, remarkably organized ultrastructure, physiological sarcomere length (2.2 µm) and density of mitochondria (30%), the presence of transverse tubules, oxidative metabolism, a positive force-frequency relationship and functional calcium handling. Electromechanical properties developed more slowly and did not achieve the stage of maturity seen in adult human myocardium. Tissue maturity was necessary for achieving physiological responses to isoproterenol and recapitulating pathological hypertrophy, supporting the utility of this tissue model for studies of cardiac development and disease.
Conflict of interest statement
The authors declare the following competing financial interests: GVN and KRB are cofounders of TARA Biosystems, a Columbia spinout commercializing the use of bioengineered human cardiac tissue for drug testing.
Figures




Comment in
-
Stem-Cell-Derived Cardiomyocytes Grow Up: Start Young and Train Harder.Cell Stem Cell. 2018 Jun 1;22(6):790-791. doi: 10.1016/j.stem.2018.05.011. Cell Stem Cell. 2018. PMID: 29859168
References
-
- Bellin M, Marchetto MC, Gage FH, Mummery CL. Induced pluripotent stem cells: the new patient? Nature reviews Molecular cell biology. 2012;13:713–726. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

