The Complex Role of Store Operated Calcium Entry Pathways and Related Proteins in the Function of Cardiac, Skeletal and Vascular Smooth Muscle Cells
- PMID: 29618985
- PMCID: PMC5872157
- DOI: 10.3389/fphys.2018.00257
The Complex Role of Store Operated Calcium Entry Pathways and Related Proteins in the Function of Cardiac, Skeletal and Vascular Smooth Muscle Cells
Abstract
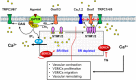
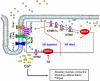
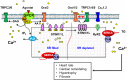
Cardiac, skeletal, and smooth muscle cells shared the common feature of contraction in response to different stimuli. Agonist-induced muscle's contraction is triggered by a cytosolic free Ca2+ concentration increase due to a rapid Ca2+ release from intracellular stores and a transmembrane Ca2+ influx, mainly through L-type Ca2+ channels. Compelling evidences have demonstrated that Ca2+ might also enter through other cationic channels such as Store-Operated Ca2+ Channels (SOCCs), involved in several physiological functions and pathological conditions. The opening of SOCCs is regulated by the filling state of the intracellular Ca2+ store, the sarcoplasmic reticulum, which communicates to the plasma membrane channels through the Stromal Interaction Molecule 1/2 (STIM1/2) protein. In muscle cells, SOCCs can be mainly non-selective cation channels formed by Orai1 and other members of the Transient Receptor Potential-Canonical (TRPC) channels family, as well as highly selective Ca2+ Release-Activated Ca2+ (CRAC) channels, formed exclusively by subunits of Orai proteins likely organized in macromolecular complexes. This review summarizes the current knowledge of the complex role of Store Operated Calcium Entry (SOCE) pathways and related proteins in the function of cardiac, skeletal, and vascular smooth muscle cells.
Keywords: Ca2+; Orai; STIM; TRPC; cardiomyocyte; skeletal muscle; vascular smooth muscle.
Figures
References
-
- Albarran L., Lopez J. J., Woodard G. E., Salido G. M., Rosado J. A. (2016). Store-operated Ca2+ entry-associated Regulatory factor (SARAF) plays an important role in the regulation of arachidonate-regulated Ca2+ (ARC) channels. J. Biol. Chem. 291, 6982–6988. 10.1074/jbc.M115.704940 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

