Mast Cells and Innate Lymphoid Cells: Underappreciated Players in CNS Autoimmune Demyelinating Disease
- PMID: 29619025
- PMCID: PMC5871669
- DOI: 10.3389/fimmu.2018.00514
Mast Cells and Innate Lymphoid Cells: Underappreciated Players in CNS Autoimmune Demyelinating Disease
Abstract
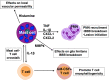
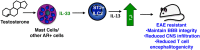
Multiple sclerosis (MS) and its mouse model, experimental autoimmune encephalomyelitis, are autoimmune CNS inflammatory diseases. As a result of a breakdown in the relatively impermeable blood-brain barrier (BBB) in affected individuals, myelin-specific CD4+ and CD8+ T cells gain entry into the immune privileged CNS and initiate myelin, oligodendrocyte, and nerve axon destruction. However, despite the absolute requirement for T cells, there is increasing evidence that innate immune cells also play critical amplifying roles in disease pathogenesis. By modulating the character and magnitude of the myelin-reactive T cell response and regulating BBB integrity, innate cells affect both disease initiation and progression. Two classes of innate cells, mast cells and innate lymphoid cells (ILCs), have been best studied in models of allergic and gastrointestinal inflammatory diseases. Yet, there is emerging evidence that these cell types also exert a profound influence in CNS inflammatory disease. Both cell types are residents within the meninges and can be activated early in disease to express a wide variety of disease-modifying cytokines and chemokines. In this review, we discuss how mast cells and ILCs can have either disease-promoting or -protecting effects on MS and other CNS inflammatory diseases and how sex hormones may influence this outcome. These observations suggest that targeting these cells and their unique mediators can be exploited therapeutically.
Keywords: experimental autoimmune encephalomyelitis; innate immunity; innate lymphoid cells; mast cells; meninges; multiple sclerosis; sex-dimorphic autoimmunity; testosterone.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

