Oxygen Availability Influences Expression of Dickeya solani Genes Associated With Virulence in Potato (Solanum tuberosum L.) and Chicory (Cichorium intybus L.)
- PMID: 29619040
- PMCID: PMC5872005
- DOI: 10.3389/fpls.2018.00374
Oxygen Availability Influences Expression of Dickeya solani Genes Associated With Virulence in Potato (Solanum tuberosum L.) and Chicory (Cichorium intybus L.)
Abstract
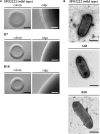
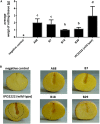
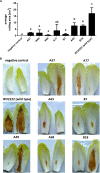
Dickeya solani is a Gram-negative necrotrophic, plant pathogenic bacterium able to cause symptoms in a variety of plant species worldwide. As a facultative anaerobe, D. solani is able to infect hosts under a broad range of oxygen concentrations found in plant environments. However, little is known about oxygen-dependent gene expression in Dickeya spp. that might contribute to its success as a pathogen. Using a Tn5 transposon, harboring a promoterless gusA reporter gene, 146 mutants of D. solani IPO2222 were identified that exhibited oxygen-regulated expression of the gene into which the insertion had occurred. Of these mutants 114 exhibited higher expression under normal oxygen conditions than hypoxic conditions while 32 were more highly expressed under hypoxic conditions. The plant host colonization potential and pathogenicity as well as phenotypes likely to contribute to the ecological fitness of D. solani, including growth rate, carbon and nitrogen source utilization, production of pectinolytic enzymes, proteases, cellulases and siderophores, swimming and swarming motility and the ability to form biofilm were assessed for 37 strains exhibiting the greatest oxygen-dependent change in gene expression. Eight mutants expressed decreased ability to cause disease symptoms when inoculated into potato tubers or chicory leaves and three of these also exhibited delayed colonization of potato plants and exhibited tissue specific differences in gene expression in these various host tissues. The genes interrupted in these eight mutants encoded proteins involved in fundamental bacterial metabolism, virulence, bacteriocin and proline transport, while three encoded hypothetical or unknown proteins. The implications of environmental oxygen concentration on the ability of D. solani to cause disease symptoms in potato are discussed.
Keywords: Tn5 transposon mutagenesis; abiotic stress; anaerobic conditions; colonization; hypoxia; virulence.
Figures
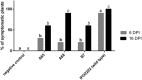
References
-
- Cardoza Y. F., Duarte V., Lopes C. A. (2016). First report of blackleg of potato caused by Dickeya solani in Brazil. Plant Dis. 101 243–243. 10.1094/PDIS-07-16-1045-PDN - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

