Bushenkangshuai Tablet Reduces Atherosclerotic Lesion by Improving Blood Lipids Metabolism and Inhibiting Inflammatory Response via TLR4 and NF- κ B Signaling Pathway
- PMID: 29619063
- PMCID: PMC5829360
- DOI: 10.1155/2018/1758383
Bushenkangshuai Tablet Reduces Atherosclerotic Lesion by Improving Blood Lipids Metabolism and Inhibiting Inflammatory Response via TLR4 and NF- κ B Signaling Pathway
Abstract
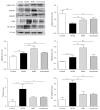
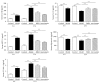
Bushenkangshuai tablet (BSKS) is a Chinese herbal compound which has been used for the treatment of cardiovascular and cerebrovascular diseases in China for decades. This study intends to explore the molecular mechanism of BSKS against atherosclerosis in ApoE-/- mice. ApoE-/- mice were fed with western-type diet for 6 weeks and then were given BSKS for 6 weeks. The results showed that BSKS attenuated the size of the atherosclerotic lesion, reduced visceral adipose content, and decreased blood lipids. We also found that BSKS promoted the expression of adiponectin and its receptors, inhibited the expression of Toll-like receptor 4 and nuclear factor-kappa B, decreased the levels of interleukin-1 beta, monocyte chemotactic protein-1, and vascular cell adhesion molecule-1, and increased the levels of interleukin-10 and adiponectin. Our data provided evidence that BSKS exerted an antiatherosclerotic effect by lowering blood lipids and inhibiting inflammatory response via TLR4 and NF-κB signaling pathway.
Figures


References
-
- Smith S. R., Lovejoy J. C., Greenway F., et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism - Clinical and Experimental. 2001;50(4):425–435. doi: 10.1053/meta.2001.21693. - DOI - PubMed
-
- Hansen T., Ahlström H., Söderberg S., et al. Visceral adipose tissue, adiponectin levels and insulin resistance are related to atherosclerosis as assessed by whole-body magnetic resonance angiography in an elderly population. Atherosclerosis. 2009;205(1):163–167. doi: 10.1016/j.atherosclerosis.2008.11.007. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

