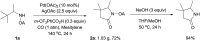Oxalyl amide assisted palladium-catalyzed synthesis of pyrrolidones via carbonylation of γ-C(sp3)-H bonds of aliphatic amine substrates
- PMID: 29619163
- PMCID: PMC5861525
- DOI: 10.1039/c5sc00519a
Oxalyl amide assisted palladium-catalyzed synthesis of pyrrolidones via carbonylation of γ-C(sp3)-H bonds of aliphatic amine substrates
Abstract
The first Pd-catalyzed regioselective γ-carbonylation of oxalyl amide protected aliphatic amines with carbon monoxide leading to synthesis of pyrrolidones has been developed. Both γ-methyl and cyclopropyl methylene C-H bonds are well activated to obtain the corresponding pyrrolidones in moderate to excellent yields. The role of 3-(trifluoromethyl)benzoic acid as an additive is critical as it helps in stabilizing the palladium intermediate formed during the catalytic cycle. The reaction scope is extended to benzylamine and allyl amine derivatives, thereby affording the corresponding products in good to excellent yields.
Figures
References
-
-
For recent reviews:
- Kakiuchi K., Murai S. Acc. Chem. Res. 2002;35:826. - PubMed
- Godula K., Sames D. Science. 2006;312:67. - PubMed
- Giri R., Shi B.-F., Engle K. M., Maugel N., Yu J.-Q. Chem. Soc. Rev. 2009;38:3242. - PubMed
- Lyons T. W., Sanford M. S. Chem. Rev. 2010;110:1147. - PMC - PubMed
- Colby D. A., Bergman R. G., Ellman J. A. Chem. Rev. 2010;110:624. - PMC - PubMed
- McMurray L., O'Hara F., Gaunt M. J. Chem. Soc. Rev. 2011;40:1885. - PubMed
- Yeung C. S., Dong V. M. Chem. Rev. 2011;111:1215. - PubMed
- Li B.-J., Shi Z.-J. Chem. Soc. Rev. 2012;41:5588. - PubMed
- Mousseau J. J., Charette A. B. Acc. Chem. Res. 2012;46:412. - PubMed
- Engle K. M., Mei T.-S., Wasa M., Yu J.-Q. Acc. Chem. Res. 2012;45:788. - PMC - PubMed
- Arockiam P. B., Bruneau C., Dixneuf P. H. Chem. Rev. 2012;112:5879. - PubMed
- Rouquet G., Chatani N. Angew. Chem., Int. Ed. 2013;52:11726. - PubMed
- Roizen J. L., Harvey M. E., Bois J. D. Acc. Chem. Res. 2012;45:911. - PMC - PubMed
- Ackermann L. Acc. Chem. Res. 2014;47:281. - PubMed
-
-
- Handbook of Organopalladium Chemistry for Organic Synthesis, ed. E.-I. Negishi, Wiley-Interscience, New York, 2002.
- Jazzar R., Hitce J., Sofack-Kreutzer J., Baudoin O. Chem.–Eur. J. 2010;16:2654. - PubMed
- Zhao Y., Jin L., Lei A. J. Am. Chem. Soc. 2008;130:9429. - PubMed
- Munday R. H., Martinelli J. R., Buchwald S. L. J. Am. Chem. Soc. 2008;130:2754. - PubMed
- Barnard C. F. J. Organometallics. 2008;27:5402.
- Brennfuhrer A., Neumann H., Beller M. Angew. Chem., Int. Ed. 2009;48:4114. - PubMed
-
-
For select examples of Pd-catalyzed carbonylation:
- Schranck J., Burhardt M., Bornschein C., Neumann H., Skrydstrup T., Beller M. Chem.–Eur. J. 2014;20:9534. - PubMed
- Chen J., Natte K., Spannenberg A., Neumann H., Langer P., Beller M., Wu X.-F. Angew. Chem., Int. Ed. 2014;53:7579. - PubMed
- Wang L., Wang Y., Liu C., Lei A. Angew. Chem., Int. Ed. 2014;53:5657. - PubMed
- Xie P., Xie Y., Qian B., Zhou H., Xia C., Huang H. J. Am. Chem. Soc. 2012;134:9902. - PubMed
- Zhang H., Shi R., Gan P., Liu C., Ding A., Wang Q., Lei A. Angew. Chem., Int. Ed. 2012;51:5204. - PubMed
- Li H., Cai G.-X., Shi Z.-J. Dalton Trans. 2010:10442. - PubMed
-
-
- Tobisu M., Chatani N., Asaumi T., Amako K., Ie Y., Fukumoto Y., Murai S. J. Am. Chem. Soc. 2000;122:12663.
- Inoue S., Shiota H., Fukumoto Y., Chatani N. J. Am. Chem. Soc. 2009;131:6898. - PubMed
- Hasegawa N., Charra V., Inoue S., Fukumoto Y., Chatani N. J. Am. Chem. Soc. 2011;133:8070. - PubMed
- Du Y., Hyster T. K., Rovis T. Chem. Commun. 2011;47:12074. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources