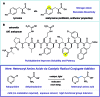
A practical and scalable system for heteroaryl amino acid synthesis
- PMID: 29619169
- PMCID: PMC5863445
- DOI: 10.1039/c7sc03612d
A practical and scalable system for heteroaryl amino acid synthesis
Abstract
A robust system for the preparation of β-heteroaryl α-amino acid derivatives has been developed using photoredox catalysis. This system operates via regiospecific activation of halogenated pyridines (or other heterocycles) and conjugate addition to dehydroalanine derivatives to deliver a wide range of unnatural amino acids. This process was conducted with good efficiency on large scale, the application of these conditions to amino ketone synthesis is shown, and a simple protocol is given for the preparation of enantioenriched amino acid synthesis, from a number of radical precursors.
Figures
Similar articles
-
Synthesis of α-Fluoro-α-Amino Acid Derivatives via Photoredox-Catalyzed Carbofluorination.ACS Catal. 2019;9(2):1558-1563. doi: 10.1021/acscatal.8b04284. Epub 2019 Jan 24. ACS Catal. 2019. PMID: 31588366 Free PMC article.
-
Photoredox Metal-Free Synthesis of Unnatural β-Silyl-α-Amino Acids via Hydrosilylation.Chem Asian J. 2023 Dec 14;18(24):e202300805. doi: 10.1002/asia.202300805. Epub 2023 Nov 9. Chem Asian J. 2023. PMID: 37906443
-
Visible-Light-Enabled Enantioconvergent Synthesis of α-Amino Acid Derivatives via Synergistic Brønsted Acid/Photoredox Catalysis.Angew Chem Int Ed Engl. 2021 Feb 23;60(9):4698-4704. doi: 10.1002/anie.202012909. Epub 2021 Jan 4. Angew Chem Int Ed Engl. 2021. PMID: 33219607
-
[Asymmetric Synthesis of Unnatural Amino Acid-containing Peptides via Direct Asymmetric Reaction of Peptidyl Compounds].Yakugaku Zasshi. 2018;138(11):1371-1379. doi: 10.1248/yakushi.18-00143. Yakugaku Zasshi. 2018. PMID: 30381645 Review. Japanese.
-
Monoterpene-based chiral β-amino acid derivatives prepared from natural sources: syntheses and applications.Amino Acids. 2011 Aug;41(3):597-608. doi: 10.1007/s00726-011-0891-5. Epub 2011 Mar 30. Amino Acids. 2011. PMID: 21448657 Review.
Cited by
-
Synthesis of amino-diamondoid pharmacophores via photocatalytic C-H aminoalkylation.Chem Commun (Camb). 2020 Aug 20;56(67):9699-9702. doi: 10.1039/d0cc02804e. Chem Commun (Camb). 2020. PMID: 32699866 Free PMC article.
-
Organometallic AlaM Reagents for Umpolung Peptide Diversification.Chem Catal. 2021 Sep 16;1(4):870-884. doi: 10.1016/j.checat.2021.05.016. Epub 2021 Jun 28. Chem Catal. 2021. PMID: 34738092 Free PMC article.
-
Dehydroamino acids: chemical multi-tools for late-stage diversification.Org Biomol Chem. 2019 Apr 10;17(15):3653-3669. doi: 10.1039/c8ob03155j. Org Biomol Chem. 2019. PMID: 30849157 Free PMC article. Review.
-
Umpolung AlaB Reagents for the Synthesis of Non-Proteogenic Amino Acids, Peptides and Proteins.Angew Chem Int Ed Engl. 2022 Aug 1;61(31):e202207153. doi: 10.1002/anie.202207153. Epub 2022 Jun 23. Angew Chem Int Ed Engl. 2022. PMID: 35653581 Free PMC article.
-
Synthesis of Spirocyclic Piperidines by Radical Hydroarylation.Synlett. 2021;32(2):211-214. doi: 10.1055/a-1315-1014. Epub 2020 Nov 19. Synlett. 2021. PMID: 34326573 Free PMC article.
References
-
- Evans D. A., Helmchen G. and Reuping M., in Asymmetric Synthesis: The Essentials, 2007, pp. 3–9.
- Gnas Y., Glorius F. Synthesis. 2006;12:1899–1930.
-
- Doyle A. G., Jacobsen E. N. Chem. Rev. 2007;107:5713–5743. - PubMed
- Davie E. A. C., Mennen S. M., Xu Y., Miller S. J. Chem. Rev. 2007;107:5759–5812. - PubMed
- Corey E. J., Helal C. J. Angew. Chem., Int. Ed. Engl. 1998;37:1986–2012. - PubMed
- Helmchen G., Pfaltz A. Acc. Chem. Res. 2000;33:336–345. - PubMed
- Zhang F.-L., Hong K., Li T.-J., Park H., Yu J.-Q. Science. 2016;351:252–256. - PMC - PubMed
- MacMillan D. W. C. Nature. 2008;455:304–308. - PubMed
- Sakthivel K., Notz W., Bui T., Barbas C. F. J. Am. Chem. Soc. 2001;123:5260–5267. - PubMed
- Bertelsen S., Jørgensen K. A. Chem. Soc. Rev. 2009;38:2178–2189. - PubMed
-
-
. For examples in synthetic peptide hormones, see:
- Blaskovich M. A. T. J. Med. Chem. 2016;59:10807–10836. - PubMed
- Reissmann T., Schally A. V., Bouchard P., Riethmuller H., Engel J. Hum. Reprod. Update. 2000;6:322–331. - PubMed
- Folkers K., Bowers C. Y., Kubiak T., Stepinski J. Biochem. Biophys. Res. Commun. 1983;111:1089–1095. - PubMed
- Asami T., Nishizawa N., Matsui H., Nishibori K., Ishibashi Y., Horikoshi Y., Nakayama M., Matsumoto S. I., Tarui N., Yamaguchi M., Matsumoto H., Ohtaki T., Kitada C. J. Med. Chem. 2013;56:8298–8307. - PubMed
-
LinkOut - more resources
Full Text Sources
Other Literature Sources