Efficient cross-effect dynamic nuclear polarization without depolarization in high-resolution MAS NMR
- PMID: 29619170
- PMCID: PMC5861987
- DOI: 10.1039/c7sc02199b
Efficient cross-effect dynamic nuclear polarization without depolarization in high-resolution MAS NMR
Abstract
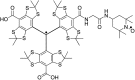
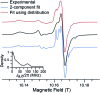
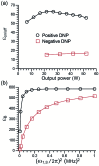
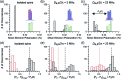
Dynamic nuclear polarization (DNP) has the potential to enhance the sensitivity of magic-angle spinning (MAS) NMR by many orders of magnitude and therefore to revolutionize atomic resolution structural analysis. Currently, the most widely used approach to DNP for studies of chemical, material, and biological systems involves the cross-effect (CE) mechanism, which relies on biradicals as polarizing agents. However, at high magnetic fields (≥5 T), the best biradicals used for CE MAS-DNP are still far from optimal, primarily because of the nuclear depolarization effects they induce. In the presence of bisnitroxide biradicals, magic-angle rotation results in a reverse CE that can deplete the initial proton Boltzmann polarization by more than a factor of 2. In this paper we show that these depolarization losses can be avoided by using a polarizing agent composed of a narrow-line trityl radical tethered to a broad-line TEMPO. Consequently, we show that a biocompatible trityl-nitroxide biradical, TEMTriPol-1, provides the highest MAS NMR sensitivity at ≥10 T, and its relative efficiency increases with the magnetic field strength. We use numerical simulations to explain the absence of depolarization for TEMTriPol-1 and its high efficiency, paving the way for the next generation of polarizing agents for DNP. We demonstrate the superior sensitivity enhancement using TEMTriPol-1 by recording the first solid-state 2D 13C-13C correlation spectrum at natural isotopic abundance at a magnetic field of 18.8 T.
Figures



Similar articles
-
Efficient Dynamic Nuclear Polarization at 800 MHz/527 GHz with Trityl-Nitroxide Biradicals.Angew Chem Int Ed Engl. 2015 Sep 28;54(40):11770-4. doi: 10.1002/anie.201504292. Epub 2015 Aug 12. Angew Chem Int Ed Engl. 2015. PMID: 26268156 Free PMC article.
-
Sensitivity analysis of magic angle spinning dynamic nuclear polarization below 6 K.J Magn Reson. 2019 Aug;305:51-57. doi: 10.1016/j.jmr.2019.05.011. Epub 2019 Jun 4. J Magn Reson. 2019. PMID: 31212198
-
Nuclear depolarization and absolute sensitivity in magic-angle spinning cross effect dynamic nuclear polarization.Phys Chem Chem Phys. 2015 Sep 14;17(34):21824-36. doi: 10.1039/c5cp03457d. Epub 2015 Aug 3. Phys Chem Chem Phys. 2015. PMID: 26235749
-
Polarizing agents and mechanisms for high-field dynamic nuclear polarization of frozen dielectric solids.Solid State Nucl Magn Reson. 2011 Sep;40(2):31-41. doi: 10.1016/j.ssnmr.2011.08.001. Epub 2011 Aug 6. Solid State Nucl Magn Reson. 2011. PMID: 21855299 Free PMC article. Review.
-
Polarizing agents for efficient high field DNP solid-state NMR spectroscopy under magic-angle spinning: from design principles to formulation strategies.Chem Sci. 2023 May 12;14(23):6120-6148. doi: 10.1039/d3sc01079a. eCollection 2023 Jun 14. Chem Sci. 2023. PMID: 37325158 Free PMC article. Review.
Cited by
-
Verdazyl-ribose: A new radical for solid-state dynamic nuclear polarization at high magnetic field.J Magn Reson. 2018 Apr;289:122-131. doi: 10.1016/j.jmr.2018.02.016. Epub 2018 Mar 1. J Magn Reson. 2018. PMID: 29501956 Free PMC article.
-
Spinning-Driven Dynamic Nuclear Polarization with Optical Pumping.J Phys Chem A. 2022 Apr 28;126(16):2600-2608. doi: 10.1021/acs.jpca.2c01559. Epub 2022 Apr 13. J Phys Chem A. 2022. PMID: 35417169 Free PMC article.
-
Highly Efficient Polarizing Agents for MAS-DNP of Proton-Dense Molecular Solids.Angew Chem Int Ed Engl. 2022 Mar 14;61(12):e202114103. doi: 10.1002/anie.202114103. Epub 2022 Feb 1. Angew Chem Int Ed Engl. 2022. PMID: 35019217 Free PMC article.
-
Sorbitol-Based Glass Matrices Enable Dynamic Nuclear Polarization beyond 200 K.J Phys Chem Lett. 2024 Aug 29;15(34):8743-8751. doi: 10.1021/acs.jpclett.4c02054. Epub 2024 Aug 20. J Phys Chem Lett. 2024. PMID: 39162721 Free PMC article.
-
Detection of the Surface of Crystalline Y2O3 Using Direct 89Y Dynamic Nuclear Polarization.J Phys Chem Lett. 2019 Jun 20;10(12):3501-3508. doi: 10.1021/acs.jpclett.9b01185. Epub 2019 Jun 12. J Phys Chem Lett. 2019. PMID: 31150249 Free PMC article.
References
-
- Ardenkjaer-Larsen J.-H., Boebinger G. S., Comment A., Duckett S., Edison A. S., Engelke F., Griesinger C., Griffin R. G., Hilty C., Maeda H., Parigi G., Prisner T., Ravera E., van Bentum J., Vega S., Webb A., Luchinat C., Schwalbe H., Frydman L. Angew. Chem., Int. Ed. 2015;54:9162–9185. - PMC - PubMed
-
- Rossini A. J., Zagdoun A., Lelli M., Lesage A., Copéret C., Emsley L. Acc. Chem. Res. 2013;46:1942–1951. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

