Deformylation reaction-based probe for in vivo imaging of HOCl
- PMID: 29619205
- PMCID: PMC5868080
- DOI: 10.1039/c7sc03784h
Deformylation reaction-based probe for in vivo imaging of HOCl
Abstract
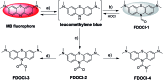
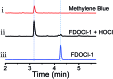
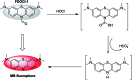
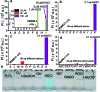
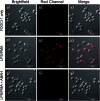
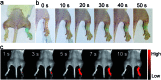
The detection of hypochlorous acid (HOCl) in vivo is vitally important because the local concentration of HOCl is highly correlated with some diseases such as atherosclerosis and rheumatoid arthritis. However, in vivo detection of HOCl remains a challenge due to the lack of a suitable probe. We report here a near-infrared (NIR) emissive "turn-on" probe (FDOCl-1) based on a methylene blue derivative, which can quickly detect HOCl via a newly found deformylation mechanism. FDOCl-1 displays remarkable selectivity and sensitivity towards HOCl. The dramatic changes in colour and NIR emission were used to detect HOCl in vitro and in vivo in a mouse arthritis model.
Figures


References
-
- Chen X., Wang F., Hyun J. Y., Wei T., Qiang J., Ren X., Shin I., Yoon J. Chem. Soc. Rev. 2016;45:2976–3016. - PubMed
-
- Yang Y., Zhao Q., Feng W., Li F. Chem. Rev. 2013;113:192–270. - PubMed
-
- Meng L., Wu Y., Yi T. Chem. Commun. 2014;50:4843–4845. - PubMed
-
- Wen Y., Liu K., Yang H., Li Y., Lan H., Liu Y., Zhang X., Yi T. Anal. Chem. 2014;86:9970–9976. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

