The Chondro-Osseous Continuum: Is It Possible to Unlock the Potential Assigned Within?
- PMID: 29619368
- PMCID: PMC5871702
- DOI: 10.3389/fbioe.2018.00028
The Chondro-Osseous Continuum: Is It Possible to Unlock the Potential Assigned Within?
Abstract
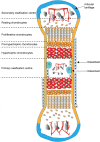
Endochondral ossification (EO), by which long bones of the axial skeleton form, is a tightly regulated process involving chondrocyte maturation with successive stages of proliferation, maturation, and hypertrophy, accompanied by cartilage matrix synthesis, calcification, and angiogenesis, followed by osteoblast-mediated ossification. This developmental sequence reappears during fracture repair and in osteoarthritic etiopathology. These similarities suggest that EO, and the cells involved, are of great clinical importance for bone regeneration as it could provide novel targeted approaches to increase specific signaling to promote fracture healing, and if regulated appropriately in the treatment of osteoarthritis. The long-held accepted dogma states that hypertrophic chondrocytes are terminally differentiated and will eventually undergo apoptosis. In this mini review, we will explore recent evidence from experiments that revisit the idea that hypertrophic chondrocytes have pluripotent capacity and may instead transdifferentiate into a specific sub-population of osteoblast cells. There are multiple lines of evidence, including our own, showing that local, selective alterations in cartilage extracellular matrix (ECM) remodeling also indelibly alter bone quality. This would be consistent with the hypothesis that osteoblast behavior in long bones is regulated by a combination of their lineage origins and the epigenetic effects of chondrocyte-derived ECM which they encounter during their recruitment. Further exploration of these processes could help to unlock potential novel targets for bone repair and regeneration and in the treatment of osteoarthritis.
Keywords: bone; cartilage; chondrocyte; extracellular matrix; osteoblast; transdifferentiation.
Figures

Similar articles
-
The art of building bone: emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification.Bone Res. 2018 Jun 14;6:19. doi: 10.1038/s41413-018-0021-z. eCollection 2018. Bone Res. 2018. PMID: 29928541 Free PMC article.
-
Chondrocyte-to-osteoblast transformation in mandibular fracture repair.J Orthop Res. 2021 Aug;39(8):1622-1632. doi: 10.1002/jor.24904. Epub 2020 Nov 18. J Orthop Res. 2021. PMID: 33140859 Free PMC article.
-
Hypertrophic chondrocytes at the junction of musculoskeletal structures.Bone Rep. 2023 Jul 3;19:101698. doi: 10.1016/j.bonr.2023.101698. eCollection 2023 Dec. Bone Rep. 2023. PMID: 37485234 Free PMC article.
-
The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification.J Endocrinol. 2011 Nov;211(2):109-21. doi: 10.1530/JOE-11-0048. Epub 2011 Jun 3. J Endocrinol. 2011. PMID: 21642379 Review.
-
Fate of the hypertrophic chondrocyte: microenvironmental perspectives on apoptosis and survival in the epiphyseal growth plate.Birth Defects Res C Embryo Today. 2005 Dec;75(4):330-9. doi: 10.1002/bdrc.20057. Birth Defects Res C Embryo Today. 2005. PMID: 16425255 Review.
Cited by
-
A Shifting Paradigm: Transformation of Cartilage to Bone during Bone Repair.J Dent Res. 2023 Jan;102(1):13-20. doi: 10.1177/00220345221125401. Epub 2022 Oct 27. J Dent Res. 2023. PMID: 36303415 Free PMC article. Review.
-
Wireless Measurements Using Electrical Impedance Spectroscopy to Monitor Fracture Healing.Sensors (Basel). 2022 Aug 19;22(16):6233. doi: 10.3390/s22166233. Sensors (Basel). 2022. PMID: 36016004 Free PMC article.
-
MMP14 cleaves PTH1R in the chondrocyte-derived osteoblast lineage, curbing signaling intensity for proper bone anabolism.Elife. 2023 Mar 9;12:e82142. doi: 10.7554/eLife.82142. Elife. 2023. PMID: 36892459 Free PMC article.
-
Mesenchymal Stromal Cells for Aging Cartilage Regeneration: A Review.Int J Mol Sci. 2024 Nov 30;25(23):12911. doi: 10.3390/ijms252312911. Int J Mol Sci. 2024. PMID: 39684619 Free PMC article. Review.
-
Cartilage-Specific Cre Recombinase Transgenes/Alleles in the Mouse.Methods Mol Biol. 2021;2245:23-38. doi: 10.1007/978-1-0716-1119-7_3. Methods Mol Biol. 2021. PMID: 33315193
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

