A Population Pharmacokinetic and Pharmacodynamic Analysis of RP5063 Phase 2 Study Data in Patients with Schizophrenia or Schizoaffective Disorder
- PMID: 29619682
- PMCID: PMC6133081
- DOI: 10.1007/s13318-018-0472-z
A Population Pharmacokinetic and Pharmacodynamic Analysis of RP5063 Phase 2 Study Data in Patients with Schizophrenia or Schizoaffective Disorder
Abstract
Background and objective: RP5063 is a novel multimodal dopamine (D)-serotonin (5-HT) stabilizer possessing partial agonist activity for D2/3/4 and 5-HT1A/2A, antagonist activity for 5-HT2B/2C/7, and moderate affinity for the serotonin transporter. Phase 2 trial data analysis of RP5063 involving patients with schizophrenia and schizoaffective disorder defined: (1) the pharmacokinetic profile; and (2) the pharmacokinetic/pharmacodynamic relationships.
Methods: Pharmacokinetic sample data (175 patients on RP5063; 28 doses/patient) were analyzed, utilized one- and two-compartment models, and evaluated the impact of covariates. Pharmacodynamic analysis involved development of an Emax model.
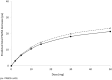
Results: The pharmacokinetic analysis identified a one-compartment model incorporating body mass index influence on volume as the optimum construct, with fixed-effect parameters: (1) oral clearance (Cl/F), 5.11 ± 0.11 L/h; (2) volume of distribution (Vc/F), 328.00 ± 31.40 L; (3) absorption constant (ka) 0.42 ± 0.17 h-1; (4) lag time (t lag) of 0.41 ± 0.02 h; and (5) a calculated half-life of 44.5 h. Pharmacokinetics were linear related to dose. An Emax model for total Positive and Negative Syndrome Scale (PANSS) scores as the response factor against cumulative area under the curve (AUC) provided fixed-effect estimates: (1) Eo = 87.3 ± 0.71 (PANSS Units; pu); (2) Emax = - 31.60 ± 4.05 (pu); and (3) AUC50 = 89.60 ± 30.10 (µg·h/mL). The predicted PANSS improvement reflected a clinical dose range of 5-30 mg.
Conclusions: Pharmacokinetics of RP5063 behaved predictably and consistently. Pharmacodynamics were characterized using an Emax model, reflecting total PANSS score as a function of cumulative AUC, that showed high predictability and low variability when correlated with actual observations.
Conflict of interest statement
Conflict of Interest
Arul Prakash, M. Pharm., Laxminarayan Bhat, PhD, and Marc Cantillon, MD are employees of Reviva Pharmaceuticals, Inc., and Robert Ings, PhD is a consultant to Reviva Pharmaceuticals, Inc.
Ethical Approval
All procedures performed in the phase 2 REFRESH trial were in accordance with the 1964 Helsinki declaration (and its amendments). This study was approved by institutional review boards for each of the investigative sites located in India, Philippines, Malaysia, Moldova, and USA. Written informed consent was obtained from participants directly, or from parents or care givers of all participants.
Figures





References
-
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5. Washington: American Psychiatric Association; 2013. Schizophrenia and other psychotic disorders; pp. 89–122.
-
- National Institute of Mental Health. Schizophrenia. 2009. Available at: http://www.nimh.nih.gov/health/publications/schizophrenia-easyto-read/ni.... Accessed 20 June 2014.
-
- ) National Institute of Mental Health. What is Schizophrenia? 2015. http://www.nimh.nih.gov/health/topics/schizophrenia/index.shtml. Accessed 4 June 2017.
-
- Opler L, Kay S, Lindenmayer JP, et al. Structured clinical interview—positive and negative syndrome scale. North Tonawanda: Multi-Health Systems; 1999. https://www.mhs.com/MHS-Assessment?prodname=panss. Accessed 4 June 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

