Adenovirus‑mediated knockdown of activin A receptor type 2A attenuates immune‑induced hepatic fibrosis in mice and inhibits interleukin‑17‑induced activation of primary hepatic stellate cells
- PMID: 29620144
- PMCID: PMC5979935
- DOI: 10.3892/ijmm.2018.3600
Adenovirus‑mediated knockdown of activin A receptor type 2A attenuates immune‑induced hepatic fibrosis in mice and inhibits interleukin‑17‑induced activation of primary hepatic stellate cells
Abstract
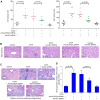
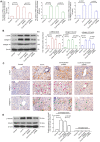
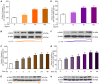
Fibrosis induces a progressive loss of liver function, thus leading to organ failure. Activins are secreted proteins that belong to the transforming growth factor (TGF)‑β superfamily, which initiate signaling by binding to their two type II receptors: Activin A receptor type 2A (ACVR2A) and activin A receptor type 2B. Previous studies that have explored the mechanisms underlying immune‑induced hepatic fibrosis have mainly focused on TGF‑β signaling, not activin signaling. To investigate the role of the activin pathway in this disease, adenovirus particles containing short hairpin (sh)RNA targeting ACVR2A mRNA (Ad‑ACVR2A shRNA) were administered to mice, which were chronically treated with concanavalin A (Con A). The pathological changes in the liver were evaluated with hematoxylin/eosin staining, Masson trichrome staining and immunohistochemical assay. The results detected an increase in serum activin A and liver ACVR2A in Con A‑treated animals. Conversely, liver function was partially restored and fibrotic injury was attenuated when activin signaling was blocked. In addition, the activation of hepatic stellate cells (HSCs) in response to Con A was suppressed by Ad‑ACVR2A shRNA, as evidenced by decreased α‑smooth muscle actin, and type I and IV collagen expression. Furthermore, primary mouse HSCs (mHSCs) were activated when exposed to interleukin (IL)‑17A or IL‑17F, which are two major cytokines produced by cluster of differentiation 4+ T helper 17 cells. The levels of activin A, type I and IV collagen were determined with ELISA kits and the expression of fibrotic molecules was determined with western blot analysis. Conversely, blocking activin/ACVR2A impaired the potency of HSCs to produce collagens in response to IL‑17s. In addition, C terminus phosphorylation of Smad2 on Ser465 and Ser467, induced by either Con A in the liver or by IL‑17s in mHSCs, was partly inhibited when activin A/ACVR2A signaling was suppressed. Collectively, the present study demonstrated an involvement of activated activin A/ACVR2A/Smad2 signaling in immune‑induced hepatic fibrosis.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

