S-phase kinase-associated protein 2 is involved in epithelial-mesenchymal transition in methotrexate-resistant osteosarcoma cells
- PMID: 29620168
- PMCID: PMC5919717
- DOI: 10.3892/ijo.2018.4345
S-phase kinase-associated protein 2 is involved in epithelial-mesenchymal transition in methotrexate-resistant osteosarcoma cells
Abstract
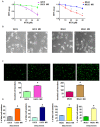
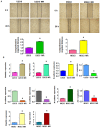
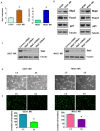
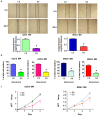
Osteosarcoma (OS), a common worldwide primary aggressive bone malignancy, arises from primitive transformed cells of mesenchymal origin and usually attacks adolescents and young adults. Methotrexate (MTX) is the anti-folate drug used as a pivotal chemotherapeutic agent in the treatment of OS. However, patients with OS often develop drug resistance, leading to poor treatment outcomes. In the present study, in order to explore the underlying mechanisms responsible for MTX resistance, we established MTX‑resistant OS cells using the U2OS and MG63 cell lines and examined whether MTX‑resistant OS cells underwent epithelial-mesenchymal transition (EMT) by Transwell assay, wound healing assay, MTT assay, RT-PCR and western blot analysis. We found that the viability of the MTX‑resistant cells remained relatively unaltered following further treatment with MTX compared to the parental cells. The resistant cells appeared to possess a mesenchymal phenotype, with an elongated and more spindle‑like shape, and acquired enhanced invasive, migratory and attachment abilities. The measurement of EMT markers also supported EMT transition in the MTX‑resistant OS cells. Our result further demonstrated that the overexpression of S-phase kinase-associated protein 2 (Skp2) was closely involved in the resistance of OS cells to MTX and in the acquirement of EMT properties. Thus, the pharmacological inhibition of Skp2 may prove to be a novel therapeutic strategy with which to overcome drug resistance in OS.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

