Increased level of cell-derived microparticles in the cyst fluids of odontogenic keratocysts
- PMID: 29620170
- PMCID: PMC5919707
- DOI: 10.3892/ijo.2018.4361
Increased level of cell-derived microparticles in the cyst fluids of odontogenic keratocysts
Abstract
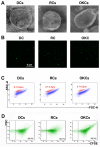
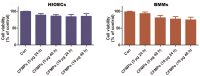
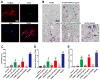
The aim of this study was to examine the level and basic characteristics of cell‑derived microparticles (MPs) in the cyst fluids of odontogenic keratocysts (OKCs). For this purpose, MPs from the cyst fluids (CFMPs) of OKCs were purified by a classic differential centrifugation method and characterized by a transmission electron microscope and fluorescence microscope. Flow cytometric analysis was used to determine the size, concentration and cellular origins of the CFMPs. Moreover, the expression level of receptor activator for nuclear factor‑κB ligand in the OKCs was evaluated by immunohistochemical staining and then analyzed for its correlation with the concentration of CFMPs by Spearman's rank correlation test. In addition, reverse transcription‑quantitative polymerase chain reaction (RT‑qPCR) and tartaric‑resistant acid phosphatase (TRAP) staining were performed to examine the osteoclastogenesis of mouse bone marrow‑derived macrophages (BMMs) in response to CFMPs. The results revealed that the levels of total CFMPs were significantly elevated in OKCs compared with dentigerous cysts (DCs) and radicular cysts (RCs). In addition, in vitro experiments further revealed that CFMPs derived from the OKCs of patients could be taken up by BMMs, leading to a significant increase in the mRNA expression levels of nuclear factor of activated T‑cells 1 (NFATc1) and TRAP. Moreover, TRAP‑positive multinucleated osteoclasts were successfully cultured in the presence of macrophage colony‑stimulating factor (M‑CSF) and CFMPs with BMMs. On the whole, our findings indicate that patients with OKCs have higher levels of CFMPs compared with patients with DCs and RCs, which may be associated with the bone resorption of OKCs.
Figures
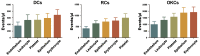




References
-
- Thompson L. World Health Organization classification of tumours: Pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006;85:74. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

