Metformin inhibits ovarian cancer via decreasing H3K27 trimethylation
- PMID: 29620187
- PMCID: PMC5919713
- DOI: 10.3892/ijo.2018.4343
Metformin inhibits ovarian cancer via decreasing H3K27 trimethylation
Abstract
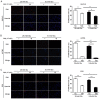
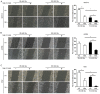
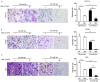
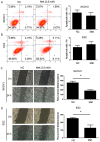
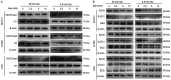
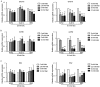
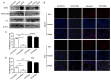
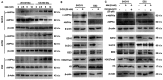
Metformin has been used for the treatment of type II diabetes mellitus for decades. Recently, used of metformin in the therapy of diverse human cancer types has received widespread attention, while the underlying mechanisms have been not fully elucidated. In the current study, 5-ethynyl-20-deoxyuridine assay to detect cell proliferation, flow cytometry to detect apoptosis, scratch wound healing and Transwell migration assay to detect cell migration capacity. The current study reported that metformin inhibited cell proliferation and migration, and promoted apoptosis in ovarian cancer cells, particularly under normoglycemic conditions in vitro. Metformin treatment significantly promoted the phosphorylation of AMP-activated protein kinase (AMPK), and reduced histone H3 lysine 27 trimethylation (H3K27me3) and polycomb repressor complex 2 (PRC2) levels. Additionally, overexpression of EZH2 to increase H3K27me3 abrogated the effect of metformin on the cell proliferation, migration and apoptosis in SKOV3 and ES2 cells. Similar to metformin, another AMPK agonist, 2-deoxy-D-glucose, reduced the H3K27me3 level and PRC2 expression. In cells pretreated with Compound C, an AMPK inhibitor, metformin was not able to induce AMPK phosphorylation or reduce H3K27me3. Metformin-mediated AMPK activation and H3K27me3 inhibition were more robust in cells exposed to low glucose (5.5 mM) compared with those exposed to high glucose (25 mM). These findings implicate H3K27me3 repression mediated by AMPK phosphorylation in the antitumor effect of metformin in ovarian cancer, indicating that metformin alters epigenetic modifications by targeting PRC2 and supports the use of metformin in treatment of patients with epithelial ovarian cancer without diabetes.
Figures



Similar articles
-
Phosphorylation of EZH2 by AMPK Suppresses PRC2 Methyltransferase Activity and Oncogenic Function.Mol Cell. 2018 Jan 18;69(2):279-291.e5. doi: 10.1016/j.molcel.2017.12.024. Mol Cell. 2018. PMID: 29351847 Free PMC article.
-
Protein kinase A-mediated phosphorylation regulates STAT3 activation and oncogenic EZH2 activity.Oncogene. 2018 Jun;37(26):3589-3600. doi: 10.1038/s41388-018-0218-z. Epub 2018 Mar 26. Oncogene. 2018. PMID: 29576612 Free PMC article.
-
H2AK119Ub1 and H3K27Me3 in molecular staging for survival prediction of patients with pancreatic ductal adenocarcinoma.Oncotarget. 2014 Nov 15;5(21):10421-33. doi: 10.18632/oncotarget.2126. Oncotarget. 2014. PMID: 25431952 Free PMC article.
-
H3K27me3 in Diffuse Midline Glioma and Epithelial Ovarian Cancer: Opposing Epigenetic Changes Leading to the Same Poor Outcomes.Cells. 2022 Oct 26;11(21):3376. doi: 10.3390/cells11213376. Cells. 2022. PMID: 36359771 Free PMC article. Review.
-
PRC2 mediated H3K27 methylations in cellular identity and cancer.Curr Opin Cell Biol. 2015 Dec;37:42-8. doi: 10.1016/j.ceb.2015.10.003. Epub 2015 Nov 11. Curr Opin Cell Biol. 2015. PMID: 26497635 Review.
Cited by
-
Loss of H3K27me3 in meningiomas.Neuro Oncol. 2021 Aug 2;23(8):1282-1291. doi: 10.1093/neuonc/noab036. Neuro Oncol. 2021. PMID: 33970242 Free PMC article.
-
Pleiotropic Effects of Metformin on Cancer.Int J Mol Sci. 2018 Sep 20;19(10):2850. doi: 10.3390/ijms19102850. Int J Mol Sci. 2018. PMID: 30241339 Free PMC article. Review.
-
Metformin Affects Olaparib Sensitivity through Induction of Apoptosis in Epithelial Ovarian Cancer Cell Lines.Int J Mol Sci. 2021 Sep 29;22(19):10557. doi: 10.3390/ijms221910557. Int J Mol Sci. 2021. PMID: 34638899 Free PMC article.
-
Metformin triggers the intrinsic apoptotic response in human AGS gastric adenocarcinoma cells by activating AMPK and suppressing mTOR/AKT signaling.Int J Oncol. 2019 Apr;54(4):1271-1281. doi: 10.3892/ijo.2019.4704. Epub 2019 Jan 30. Int J Oncol. 2019. PMID: 30720062 Free PMC article.
-
Metformin Reduces Histone H3K4me3 at the Promoter Regions of Positive Cell Cycle Regulatory Genes in Lung Cancer Cells.Cancers (Basel). 2021 Feb 10;13(4):739. doi: 10.3390/cancers13040739. Cancers (Basel). 2021. PMID: 33578894 Free PMC article.
References
-
- Dowling RJ, Niraula S, Chang MC, Done SJ, Ennis M, McCready DR, Leong WL, Escallon JM, Reedijk M, Goodwin PJ, et al. Changes in insulin receptor signaling underlie neoadjuvant metformin administration in breast cancer: A prospective window of opportunity neoadjuvant study. Breast Cancer Res. 2015;17:32. doi: 10.1186/s13058-015-0540-0. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

