Enrichment and mutation detection of circulating tumor cells from blood samples
- PMID: 29620284
- PMCID: PMC5983925
- DOI: 10.3892/or.2018.6342
Enrichment and mutation detection of circulating tumor cells from blood samples
Abstract
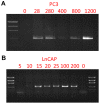
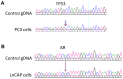
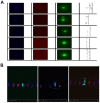
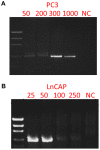
The potential of circulating tumor cells (CTCs) in the diagnosis and prognosis of cancer patients has become increasingly attractive. However, molecular analysis of CTCs is hindered by low sensitivity and a high level of background leukocytes in CTC enrichment technologies. We have developed a novel protocol using a microfluidic device, which enriches and retrieves CTCs from blood samples. The principle of CTC capturing is that tumor cells are larger and less deformable than normal blood cells. To evaluate the potential of utilizing Celsee PREP100 in CTC molecular analysis, we prepared prostate cancer cell lines PC3 and LNCaP, retrieved the captured cells and analyzed them using PCR amplicon sequencing. We were able to recover an average of 79% of 110‑1,100 PC3 and 60‑1,500 LNCaP cells, and detect the p.K139fs*3 deletion of the p53 gene in PC3 cells and p.T877A mutation of the androgen receptor gene in LNCaP cells. Next, we spiked these two types of cells into normal donor blood samples, captured the cells and analyzed them using PCR amplicon sequencing. The PC3 and LNCaP cells were captured and retrieved with the ratio of captured CTCs to the background leukocytes reaching 1:1.5 for PC3 and 1:2.9 for LNCaP cells. We further revealed that the p.K139fs*3 deletion and p.T877A mutation can be detected in the captured PC3 and LNCaP cells, respectively. We successfully validated this approach using clinical blood samples from patients with metastatic prostate cancer. Our results demonstrated a novel approach for CTC enrichment and illustrated the potential of CTC molecular characterization for diagnosis, prognosis and treatment selection of patients with metastatic malignancy.
Figures

References
-
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

