Preclinical efficacy studies in investigator brochures: Do they enable risk-benefit assessment?
- PMID: 29621228
- PMCID: PMC5886385
- DOI: 10.1371/journal.pbio.2004879
Preclinical efficacy studies in investigator brochures: Do they enable risk-benefit assessment?
Abstract
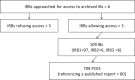
Human protection policies require favorable risk-benefit judgments prior to launch of clinical trials. For phase I and II trials, evidence for such judgment often stems from preclinical efficacy studies (PCESs). We undertook a systematic investigation of application materials (investigator brochures [IBs]) presented for ethics review for phase I and II trials to assess the content and properties of PCESs contained in them. Using a sample of 109 IBs most recently approved at 3 institutional review boards based at German Medical Faculties between the years 2010-2016, we identified 708 unique PCESs. We then rated all identified PCESs for their reporting on study elements that help to address validity threats, whether they referenced published reports, and the direction of their results. Altogether, the 109 IBs reported on 708 PCESs. Less than 5% of all PCESs described elements essential for reducing validity threats such as randomization, sample size calculation, and blinded outcome assessment. For most PCESs (89%), no reference to a published report was provided. Only 6% of all PCESs reported an outcome demonstrating no effect. For the majority of IBs (82%), all PCESs were described as reporting positive findings. Our results show that most IBs for phase I/II studies did not allow evaluators to systematically appraise the strength of the supporting preclinical findings. The very rare reporting of PCESs that demonstrated no effect raises concerns about potential design or reporting biases. Poor PCES design and reporting thwart risk-benefit evaluation during ethical review of phase I/II studies.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: JK serves in a remunerative capacity on the data safety monitoring board of an early-phase trial being pursued by Dimension Therapeutics. JK is part of the Editorial Board of the Meta-Research section of
Figures
References
-
- Kimmelman J. A theoretical framework for early human studies: uncertainty, intervention ensembles, and boundaries. Trials. 2012;13:173 doi: 10.1186/1745-6215-13-173 ; PubMed Central PMCID: PMC3551836. - DOI - PMC - PubMed
-
- Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010;8(3):e1000344 doi: 10.1371/journal.pbio.1000344 ; PubMed Central PMCID: PMC2846857. - DOI - PMC - PubMed
-
- Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature. 2012;483(7391):531–3. doi: 10.1038/483531a . - DOI - PubMed
-
- ter Riet G, Korevaar DA, Leenaars M, Sterk PJ, Van Noorden CJ, Bouter LM, et al. Publication bias in laboratory animal research: a survey on magnitude, drivers, consequences and potential solutions. PLoS ONE. 2012;7(9):e43404 doi: 10.1371/journal.pone.0043404 ; PubMed Central PMCID: PMC3434185. - DOI - PMC - PubMed
-
- Kilkenny C, Parsons N, Kadyszewski E, Festing MF, Cuthill IC, Fry D, et al. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS One. 2009;4(11):e7824 doi: 10.1371/journal.pone.0007824 ; PubMed Central PMCID: PMC2779358. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical