Mapping human pluripotent stem cell differentiation pathways using high throughput single-cell RNA-sequencing
- PMID: 29622030
- PMCID: PMC5887227
- DOI: 10.1186/s13059-018-1426-0
Mapping human pluripotent stem cell differentiation pathways using high throughput single-cell RNA-sequencing
Abstract
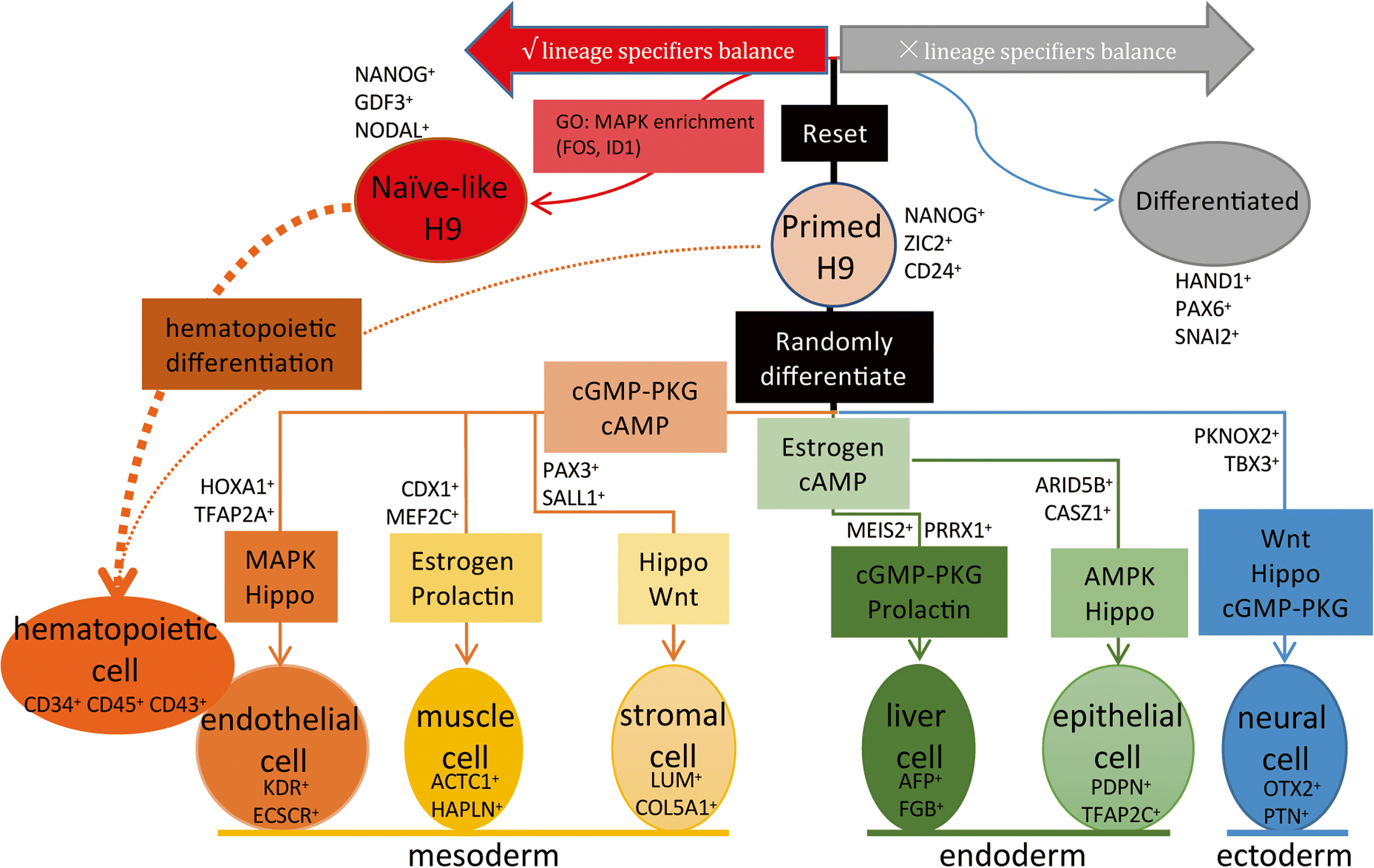
Background: Human pluripotent stem cells (hPSCs) provide powerful models for studying cellular differentiations and unlimited sources of cells for regenerative medicine. However, a comprehensive single-cell level differentiation roadmap for hPSCs has not been achieved.
Results: We use high throughput single-cell RNA-sequencing (scRNA-seq), based on optimized microfluidic circuits, to profile early differentiation lineages in the human embryoid body system. We present a cellular-state landscape for hPSC early differentiation that covers multiple cellular lineages, including neural, muscle, endothelial, stromal, liver, and epithelial cells. Through pseudotime analysis, we construct the developmental trajectories of these progenitor cells and reveal the gene expression dynamics in the process of cell differentiation. We further reprogram primed H9 cells into naïve-like H9 cells to study the cellular-state transition process. We find that genes related to hemogenic endothelium development are enriched in naïve-like H9. Functionally, naïve-like H9 show higher potency for differentiation into hematopoietic lineages than primed cells.
Conclusions: Our single-cell analysis reveals the cellular-state landscape of hPSC early differentiation, offering new insights that can be harnessed for optimization of differentiation protocols.
Keywords: Embryoid body; Naïve human pluripotent stem cell; Primed human pluripotent stem cell; Single-cell RNA-sequencing.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. 10.1038/nbt1393. - PubMed
Publication types
MeSH terms
Grants and funding
- 81770188/National Natural Science Foundation of China/International
- 31722027/National Natural Science Foundation of China/International
- 31701290/National Natural Science Foundation of China/International
- 2016XZZX002-04/Fundamental Research Funds for the Central Universities/International
- 2017YFA0103401/National Key Program on Stem Cell and Translational Research/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

