Myeloid Conditioning with c-kit-Targeted CAR-T Cells Enables Donor Stem Cell Engraftment
- PMID: 29622475
- PMCID: PMC5993968
- DOI: 10.1016/j.ymthe.2018.03.003
Myeloid Conditioning with c-kit-Targeted CAR-T Cells Enables Donor Stem Cell Engraftment
Abstract
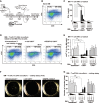
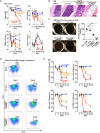
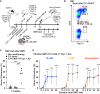
We report a novel approach to bone marrow (BM) conditioning using c-kit-targeted chimeric antigen receptor T (c-kit CAR-T) cells in mice. Previous reports using anti-c-kit or anti-CD45 antibody linked to a toxin such as saporin have been promising. We developed a distinctly different approach using c-kit CAR-T cells. Initial studies demonstrated in vitro killing of hematopoietic stem cells by c-kit CAR-T cells but poor expansion in vivo and poor migration of CAR-T cells into BM. Pre-treatment of recipient mice with low-dose cyclophosphamide (125 mg/kg) together with CXCR4 transduction in the CAR-T cells enhanced trafficking to and expansion in BM (<1%-13.1%). This resulted in significant depletion of the BM c-kit+ population (9.0%-0.1%). Because congenic Thy1.1 CAR-T cells were used in the Thy1.2-recipient mice, anti-Thy1.1 antibody could be used to deplete CAR-T cells in vivo before donor BM transplant. This achieved 20%-40% multilineage engraftment. We applied this conditioning to achieve an average of 28% correction of chronic granulomatous disease mice by wild-type BM transplant. Our findings provide a proof of concept that c-kit CAR-T cells can achieve effective BM conditioning without chemo-/radiotherapy. Our work also demonstrates that co-expression of a trafficking receptor can enhance targeting of CAR-T cells to a designated tissue.
Keywords: CAR-T cells; CXCR4; c-kit; hematopoietic stem cell transplantation; immunotherapy.
Published by Elsevier Inc.
Figures



Comment in
-
Next-Generation Conditioning for Bone Marrow Transplantation: Paving the Way for CAR-T Cell-Based Conditioning.Mol Ther. 2018 May 2;26(5):1167-1168. doi: 10.1016/j.ymthe.2018.04.011. Mol Ther. 2018. PMID: 29685383 Free PMC article. No abstract available.
References
-
- Heimall J., Puck J., Buckley R., Fleisher T.A., Gennery A.R., Neven B., Slatter M., Haddad E., Notarangelo L.D., Baker K.S. Current Knowledge and Priorities for Future Research in Late Effects after Hematopoietic Stem Cell Transplantation (HCT) for Severe Combined Immunodeficiency Patients: A Consensus Statement from the Second Pediatric Blood and Marrow Transplant Consortium International Conference on Late Effects after Pediatric HCT. Biol. Blood Marrow Transplant. 2017;23:379–387. - PMC - PubMed
-
- De Ravin S.S., Li L., Wu X., Choi U., Allen C., Koontz S., Lee J., Theobald-Whiting N., Chu J., Garofalo M. CRISPR-Cas9 gene repair of hematopoietic stem cells from patients with X-linked chronic granulomatous disease. Sci. Transl. Med. 2017;9:eaah3480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

