Triggered recruitment of ESCRT machinery promotes endolysosomal repair
- PMID: 29622626
- PMCID: PMC6195421
- DOI: 10.1126/science.aar5078
Triggered recruitment of ESCRT machinery promotes endolysosomal repair
Abstract
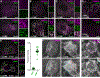
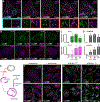
Endolysosomes can be damaged by diverse materials. Terminally damaged compartments are degraded by lysophagy, but pathways that repair salvageable organelles are poorly understood. Here we found that the endosomal sorting complex required for transport (ESCRT) machinery, known to mediate budding and fission on endolysosomes, also plays an essential role in their repair. ESCRTs were rapidly recruited to acutely injured endolysosomes through a pathway requiring calcium and ESCRT-activating factors that was independent of lysophagy. We used live-cell imaging to demonstrate that ESCRTs responded to small perforations in endolysosomal membranes and enabled compartments to recover from limited damage. Silica crystals that disrupted endolysosomes also triggered ESCRT recruitment. ESCRTs thus provide a defense against endolysosomal damage likely to be relevant in physiological and pathological contexts.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures





Comment in
-
ESCRTs offer repair service.Science. 2018 Apr 6;360(6384):33-34. doi: 10.1126/science.aat2630. Science. 2018. PMID: 29622643 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

