Isolation of a multipotent mesenchymal stem cell-like population from human adrenal cortex
- PMID: 29622661
- PMCID: PMC5919938
- DOI: 10.1530/EC-18-0067
Isolation of a multipotent mesenchymal stem cell-like population from human adrenal cortex
Abstract
Background: The highly plastic nature of adrenal cortex suggests the presence of adrenocortical stem cells (ACSC), but the exact in vivo identity of ACSC remains elusive. A few studies have demonstrated the differentiation of adipose or bone marrow-derived mesenchymal stem cells (MSC) into steroid-producing cells. We therefore investigated the isolation of multipotent MSC from human adrenal cortex.
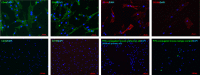
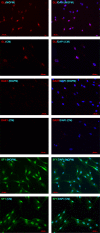
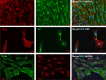
Methods: Human adrenals were obtained as discarded surgical material. Single-cell suspensions from human adrenal cortex (n = 3) were cultured onto either complete growth medium (CM) or MSC growth promotion medium (MGPM) in hypoxic condition. Following ex vivo expansion, their multilineage differentiation capacity was evaluated. Phenotype markers were analysed by immunocytochemistry and flow cytometry for cell-surface antigens associated with bone marrow MSCs and adrenocortical-specific phenotype. Expression of mRNAs for pluripotency markers was assessed by q-PCR.
Results: The formation of colony-forming unit fibroblasts comprising adherent cells with fibroblast-like morphology were observed from the monolayer cell culture, in both CM and MGPM. Cells derived from MGPM revealed differentiation towards osteogenic and adipogenic cell lineages. These cells expressed cell-surface MSC markers (CD44, CD90, CD105 and CD166) but did not express the haematopoietic, lymphocytic or HLA-DR markers. Flow cytometry demonstrated significantly higher expression of GLI1 in cell population harvested from MGPM, which were highly proliferative. They also exhibited increased expression of the pluripotency markers.
Conclusion: Our study demonstrates that human adrenal cortex harbours a mesenchymal stem cell-like population. Understanding the cell biology of adrenal cortex- derived MSCs will inform regenerative medicine approaches in autoimmune Addison's disease.
Keywords: adrenocortical stem cell; autoimmune Addison’s disease; mesenchymal stem cells; tissue regeneration.
© 2018 The authors.
Figures






References
-
- Pearce SH, Mitchell AL, Bennett S, King P, Chandran S, Nag S, Chen S, Smith BR, Issacs JD, Vaidya B. Adrenal steroidogenesis after B lymphocyte depletion therapy in new-onset Addison’s disease. Journal of Clinical Endocrinology and Metabolism 2012. 97 E1927–E1932. ( 10.1210/jc.2012-1680) - DOI - PMC - PubMed
-
- Gan EH, MacArthur K, Mitchell AL, Hughes BA, Perros P, Ball SG, James RA, Quinton R, Chen S, Furmaniak J, et al Residual adrenal function in autoimmune Addison’s disease: improvement after tetracosactide (ACTH1-24) treatment. Journal of Clinical Endocrinology and Metabolism 2014. 99 111–118. ( 10.1210/jc.2013-2449) - DOI - PubMed
-
- Ingle DJ, Higgins G. Regeneration of the adrenal gland following enucleation. American Journal of the Medical Sciences 1938. 196 232–239. ( 10.1097/00000441-193808000-00012) - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

