Pharmacology behind Common Drug Nephrotoxicities
- PMID: 29622670
- PMCID: PMC6302342
- DOI: 10.2215/CJN.00150118
Pharmacology behind Common Drug Nephrotoxicities
Abstract
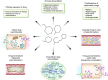
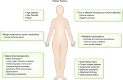
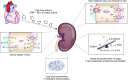
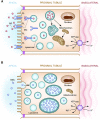
Patients are exposed to numerous prescribed and over-the-counter medications. Unfortunately, drugs remain a relatively common cause of acute and chronic kidney injury. A combination of factors including the innate nephrotoxicity of drugs, underlying patient characteristics that increase their risk for kidney injury, and the metabolism and pathway of excretion by the kidneys of the various agents administered enhance risk for drug-induced nephrotoxicity. This paper will review these clinically relevant aspects of drug-induced nephrotoxicity for the clinical nephrologist.
Keywords: Acute Kidney Injury; Drug Transporters; Humans; Nephrologists; Nonprescription Drugs; Pharmacology; Proximal Tubulopathy; Renal Elimination; Risk; acute renal failure; drug nephrotoxicity; kidney; metabolism.
Copyright © 2018 by the American Society of Nephrology.
Figures

References
-
- Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, Paganini EP, Chertow GM; Program to Improve Care in Acute Renal Disease: Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int 66: 1613–1621, 2004 - PubMed
-
- Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani V, Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators: Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 - PubMed
-
- Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA: Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med 41: 1411–1423, 2015 - PubMed
-
- Perazella MA: Drug use and nephrotoxicity in the intensive care unit. Kidney Int 81: 1172–1178, 2012 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

