An intersubunit electrostatic interaction in the GABAA receptor facilitates its responses to benzodiazepines
- PMID: 29622679
- PMCID: PMC5971448
- DOI: 10.1074/jbc.RA118.002128
An intersubunit electrostatic interaction in the GABAA receptor facilitates its responses to benzodiazepines
Abstract
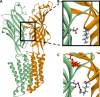
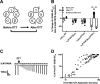
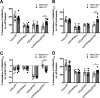
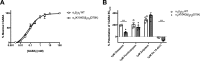
Benzodiazepines are positive allosteric modulators of the GABAA receptor (GABAAR), acting at the α-γ subunit interface to enhance GABAAR function. GABA or benzodiazepine binding induces distinct conformational changes in the GABAAR. The molecular rearrangements in the GABAAR following benzodiazepine binding remain to be fully elucidated. Using two molecular models of the GABAAR, we identified electrostatic interactions between specific amino acids at the α-γ subunit interface that were broken by, or formed after, benzodiazepine binding. Using two-electrode voltage clamp electrophysiology in Xenopus laevis oocytes, we investigated these interactions by substituting one or both amino acids of each potential pair. We found that Lys104 in the α1 subunit forms an electrostatic bond with Asp75 of the γ2 subunit after benzodiazepine binding and that this bond stabilizes the positively modified state of the receptor. Substitution of these two residues to cysteine and subsequent covalent linkage between them increased the receptor's sensitivity to low GABA concentrations and decreased its response to benzodiazepines, producing a GABAAR that resembles a benzodiazepine-bound WT GABAAR. Breaking this bond restored sensitivity to GABA to WT levels and increased the receptor's response to benzodiazepines. The α1 Lys104 and γ2 Asp75 interaction did not play a role in ethanol or neurosteroid modulation of GABAAR, suggesting that different modulators induce different conformational changes in the receptor. These findings may help explain the additive or synergistic effects of modulators acting at the GABAAR.
Keywords: Cys-loop receptor; GABA receptor; Xenopus; allosteric regulation; benzodiazepines; cysteine-mediated cross-linking; electrophysiology; electrostatics; ionotropic receptor; sedative.
© 2018 Pflanz et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
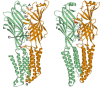




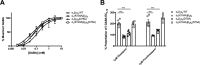
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

