Mitochondrial proteostasis in the context of cellular and organismal health and aging
- PMID: 29622680
- PMCID: PMC6462515
- DOI: 10.1074/jbc.TM117.000893
Mitochondrial proteostasis in the context of cellular and organismal health and aging
Abstract
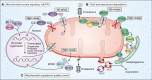
As a central hub of cellular metabolism and signaling, the mitochondrion is a crucial organelle whose dysfunction can cause disease and whose activity is intimately connected to aging. We review how the mitochondrial network maintains proteomic integrity, how mitochondrial proteotoxic stress is communicated and resolved in the context of the entire cell, and how mitochondrial systems function in the context of organismal health and aging. A deeper understanding of how mitochondrial protein quality control mechanisms are coordinated across these distinct biological levels should help explain why these mechanisms fail with age and, ultimately, how routes to intervention might be attained.
Keywords: aging; mitochondria; proteostasis; stress; unfolded protein response (UPR).
© 2019 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Pagliarini D. J., Calvo S. E., Chang B., Sheth S. A., Vafai S. B., Ong S.-E., Walford G. A., Sugiana C., Boneh A., Chen W. K., Hill D. E., Vidal M., Evans J. G., Thorburn D. R., Carr S. A., and Mootha V. K. (2008) A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 - PMC - PubMed
-
- Dick F. D. (2006) Parkinson's disease and pesticide exposures. Br. Med. Bull. 79–80, 219–231 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

